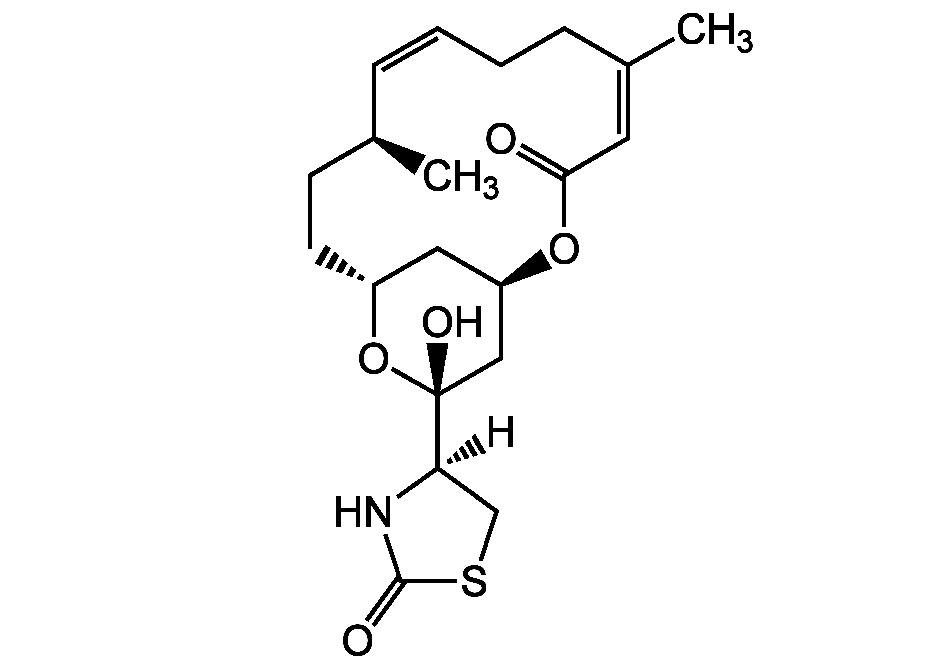
Chemical Structure
16-epi-Latrunculin B [444911-05-1]

AG-CN2-0034
Overview
- SupplierAdipoGen Life Sciences
- Product Name16-epi-Latrunculin B [444911-05-1]
- Delivery Days Customer10
- CAS Number444911-05-1
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC20H29NO5S
- Molecular Weight395.5
- Scientific DescriptionAntiviral (against herpes simplex type 1 virus (HSV-1)) [1]. Cytotoxic [1, 5]. Depolymerizes actin filaments (F-actin) [2, 3, 4]. - Chemical. CAS: 444911-05-1. Formula: C20H29NO5S. MW: 395.5. Isolated from marine sponge Negombata magnifica. Antiviral (against herpes simplex type 1 virus (HSV-1)). Cytotoxic. Depolymerizes actin filaments (F-actin).
- SMILES[H][C@@]1(CSC(=O)N1)[C@@]1(O)C[C@H]2C[C@@H](CC[C@H](C)\C=C/CC\C(C)=C/C(=O)O2)O1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Toward computing relative configurations: 16-epi-latrunculin B, a new stereoisomer of the actin polymerization inhibitor latrunculin B: T.R. Hoye, et al.; JACS 124, 7405 (2002) Abstract
- A new dimension to the biosynthetic products isolated from the sponge Negombata magnifica: B. Vilozny, et al.; J. Nat. Prod. 67, 1055 (2004)
- Diverted total synthesis: Preparation of a focused library of latrunculin analogues and evaluation of their actin-binding properties: A. Fürstner, et al.; PNAS 102, 8103 (2005)
- Total syntheses of the actin-binding macrolides latrunculin A, B, C, M, S and 16-epi-latrunculin B: A. Fürstner, et al.; Chemistry 13, 115 (2007)
- Interrogating the Bioactive Pharmacophore of the Latrunculin Chemotype by Investigating the Metabolites of Two Taxonomically Unrelated Sponges: T. Amagata, et al.; J. Med. Chem. 51, 7234 (2008)
