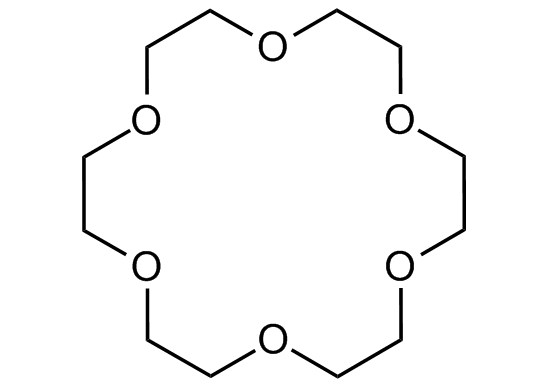
Chemical Structure
18-Crownether-6 [17455-13-9] [17455-13-9]
CDX-C0143
CAS Number17455-13-9
Product group Chemicals
Estimated Purity>99%
Molecular Weight264.32
Overview
- SupplierChemodex
- Product Name18-Crownether-6 [17455-13-9] [17455-13-9]
- Delivery Days Customer10
- CAS Number17455-13-9
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationWarning
- Molecular FormulaC12H24O6
- Molecular Weight264.32
- Scientific Description18-Crownether-6 (18-crown-6) is ionophoric and used as an efficient phase transfer catalyst and as a complexing agent with a variety of small cations. It is involved in the synthesis of diaryl ethers, diaryl thioethers and diarylamines mediated by potassium fluoride-alumina and 18-crown-6. Used to solubilizing simple metal salts in nonpolar and dipolar aprotic solvents where solvation of the anionic portion of the salt should be minimal. It facilitates the solubility of potassium permanganate in benzene, which is used for oxidizing the organic compounds. It is used to accelerate various substitution reactions as well as enhances the power of nucleophiles such as potassium acetate. It is utilized in the alkylation reactions in the presence of potassium carbonate, N-alkylation of glutarimide and succinimide with dimethylcarbonate. The complex formed by its reaction with potassium cyanide acts as a catalyst in the cyanosilylation of aldehydes, ketones and quinines with trimethylsilyl cyanide (TMSCN). - Chemical. CAS: 17455-13-9. Formula: C12H24O6. Molecular Weight: 264.32. 18-Crownether-6 (18-crown-6) is ionophoric and used as an efficient phase transfer catalyst and as a complexing agent with a variety of small cations. It is involved in the synthesis of diaryl ethers, diaryl thioethers and diarylamines mediated by potassium fluoride-alumina and 18-crown-6. Used to solubilizing simple metal salts in nonpolar and dipolar aprotic solvents where solvation of the anionic portion of the salt should be minimal. It facilitates the solubility of potassium permanganate in benzene, which is used for oxidizing the organic compounds. It is used to accelerate various substitution reactions as well as enhances the power of nucleophiles such as potassium acetate. It is utilized in the alkylation reactions in the presence of potassium carbonate, N-alkylation of glutarimide and succinimide with dimethylcarbonate. The complex formed by its reaction with potassium cyanide acts as a catalyst in the cyanosilylation of aldehydes, ketones and quinines with trimethylsilyl cyanide (TMSCN).
- SMILESC1OCCOCCOCCOCCOCCOC1
- Storage InstructionRT
- UNSPSC12352200
