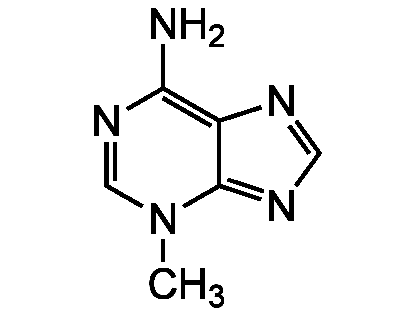
Chemical Structure
3-Methyladenine [5142-23-4]
AG-CR1-3597
CAS Number5142-23-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight149.2
Overview
- SupplierAdipoGen Life Sciences
- Product Name3-Methyladenine [5142-23-4]
- Delivery Days Customer10
- CAS Number5142-23-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC6H7N5
- Molecular Weight149.2
- Scientific DescriptionChemical. CAS: 5142-23-4. Formula: C6H7N5. MW: 149.2. Potent cell permeable and selective inhibitor of phosphatidyl-inositol 3-kinase (PI3K). Blocks class I, class II and class III PI3Ks, including some downstream targets. Blocks class I PI3K persistently and class III PI3K transiently. Induces autophagy under nutrient-rich conditions and inhibits starvation-induced autophagy due to differential effects on class I versus class III PI3 kinase. Shows some limited Vps34 preference in vitro compared to PtdIns3Kgamma. However, it is typically employed in cellular studies at a concentration of 10 mM, which can inhibit all PtdIns3Ks. Can target other kinases and affect other cellular processes, such as glycogen metabolism, lysosomal acidification, endocytosis and mitochondrial permeability transition. Anticancer compound. Neuroprotective. PKA-activation dependent lipolytic agent. Enhances ATGL-dependent hydrolysis of triacylglycerols. - Potent cell permeable and selective inhibitor of phosphatidyl-inositol 3-kinase (PI3K) [1, 7, 8]. Blocks class I, class II and class III PI3Ks, including some downstream targets [8]. Blocks class I PI3K persistently and class III PI3K transiently [7]. Induces autophagy under nutrient-rich conditions and inhibits starvation-induced autophagy due to differential effects on class I versus class III PI3 kinase [5, 7]. Shows some limited Vps34 preference in vitro compared to PtdIns3Kgamma. However, it is typically employed in cellular studies at a concentration of 10 mM, which can inhibit all PtdIns3Ks [8]. Can target other kinases and affect other cellular processes, such as glycogen metabolism, lysosomal acidification, endocytosis and mitochondrial permeability transition [2-4]. Anticancer compound [6, 10, 12]. Neuroprotective [9, 11]. PKA-activation dependent lipolytic agent. Enhances ATGL-dependent hydrolysis of triacylglycerols [13].
- SMILESCN1C=NC(N)=C2N=CN=C12
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

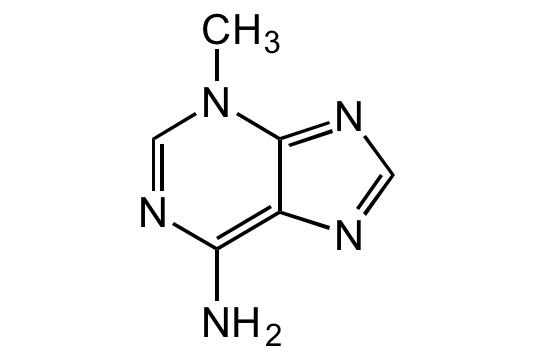


![3-Methyladenine [5142-23-4]](https://www.targetmol.com/group3/M00/38/04/CgoaEWayVUGEOywJAAAAAOs12fQ012.png)