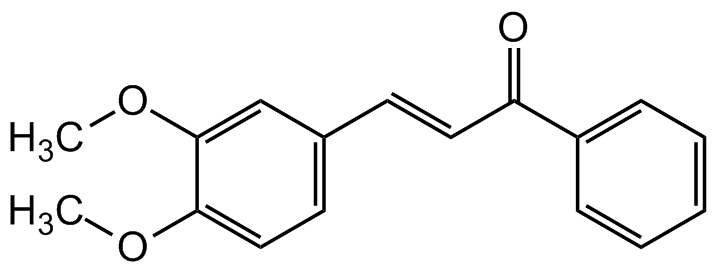
Chemical Structure
3,4-Dimethoxychalcone [5416-71-7]

AG-CN2-0531
Overview
- SupplierAdipoGen Life Sciences
- Product Name3,4-Dimethoxychalcone [5416-71-7]
- Delivery Days Customer10
- CAS Number5416-71-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC17H16O3
- Molecular Weight268.3
- Scientific DescriptionCaloric restriction mimetic (CRM). Caloric restriction mimetics (CRMs) are natural or synthetic compounds that mimic the health-promoting and longevity-extending effects of caloric restriction. CRMs provoke the deacetylation of cellular proteins coupled to an increase in autophagic flux in the absence of toxicity. Induces the deacetylation of cytoplasmic proteins and stimulates autophagic flux, requiring transcription factor EB (TFEB)- and E3 (TFE3)-dependent gene transcription and mRNA translation to trigger autophagy. Stimulates the translocation of TFEB and TFE3 into nuclei both in vitro and in vivo, in hepatocytes and cardiomyocytes. Consequently induces autophagy in vitro and in vivo, mediates autophagy-dependent cardioprotective effects and improves the efficacy of anticancer chemotherapy in vivo. So far this chalcone polyphenol has been used as an intermediate for synthesis of biochemical substances and was described to have weak antioxidant and antimicrobial activity. - Chemical. CAS: 5416-71-7. Formula: C17H16O3. MW: 268.3. Synthetic. Caloric restriction mimetic (CRM). Caloric restriction mimetics (CRMs) are natural or synthetic compounds that mimic the health-promoting and longevity-extending effects of caloric restriction. CRMs provoke the deacetylation of cellular proteins coupled to an increase in autophagic flux in the absence of toxicity. Induces the deacetylation of cytoplasmic proteins and stimulates autophagic flux, requiring transcription factor EB (TFEB)- and E3 (TFE3)-dependent gene transcription and mRNA translation to trigger autophagy. Stimulates the translocation of TFEB and TFE3 into nuclei both in vitro and in vivo, in hepatocytes and cardiomyocytes. Consequently induces autophagy in vitro and in vivo, mediates autophagy-dependent cardioprotective effects and improves the efficacy of anticancer chemotherapy in vivo. So far this chalcone polyphenol has been used as an intermediate for synthesis of biochemical substances and was described to have weak antioxidant and antimicrobial activity.
- SMILESCOC1=CC=C(C=C1OC)/C=C/C(C2=CC=CC=C2)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
