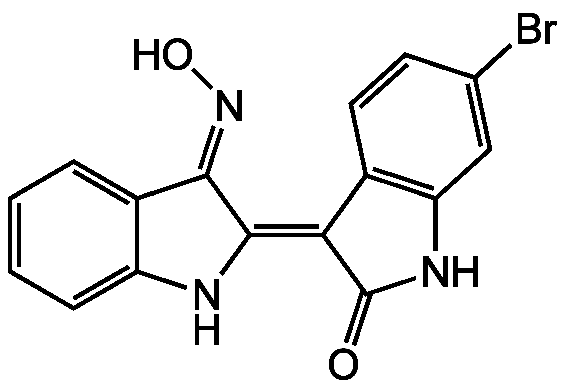
Chemical Structure
6BIO [667463-62-9]

AG-MR-C0019
Overview
- SupplierAdipoGen Life Sciences
- Product Name6BIO [667463-62-9]
- Delivery Days Customer10
- CAS Number667463-62-9
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationWarning
- Molecular FormulaC16H10BrN3O2
- Molecular Weight356.2
- Scientific DescriptionChemical. CAS: 667463-62-9. Formula: C16H10BrN3O2. MW: 356.2. Potent, reversible and ATP-competitive glycogen synthase kinase-3alpha/beta (GSK-3alpha/beta) inhibitor. JAK/STAT3 signaling inhibitor. Phosphoinositide-dependent kinase 1 (PDK1) inhibitor. Anticancer compound. Potent antiproliferative agent. Suppresses metastasis. Apoptosis inducer. Sustains pluripotency and self-renewal of human and mouse embryonic stem cells (ESCs) by activation of the Wnt signaling pathway. Anti-leishmanial (IC50 = 0.150microM). Proto-oncogene tyrosine-protein kinase receptor RET inhibitor (IC50=0.51microM). Inhibits HIV-1 transcription and protects against Tat induced neurotoxicity. - Potent, reversible and ATP-competitive glycogen synthase kinase-3alpha/beta (GSK-3alpha/beta) inhibitor. JAK/STAT3 signaling inhibitor. Phosphoinositide-dependent kinase 1 (PDK1) inhibitor. Anticancer compound. Potent antiproliferative agent. Suppresses metastasis. Apoptosis inducer. Sustains pluripotency and self-renewal of human and mouse embryonic stem cells (ESCs) by activation of the Wnt signaling pathway. Anti-leishmanial (IC50 = 0.150microM). Proto-oncogene tyrosine-protein kinase receptor RET inhibitor (IC50=0.51microM). Inhibits HIV-1 transcription and protects against Tat induced neurotoxicity.
- SMILESO\N=C1\C(\NC2=CC=CC=C\12)=C1\C(=O)NC2=C1C=CC(Br)=C2
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC51202000
References
- GSK-3-selective inhibitors derived from Tyrian purple indirubins: L. Meijer, et al.; Chem. Biol. 10, 1255 (2003)
- Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases: P. Polychronopoulos, et al.; J. Med. Chem. 47, 935 (2004)
- Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor: N. Sato, et al.; Nat. Med. 10, 55 (2004)
- The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes: A.S. Tseng, et al.; Chem. Biol. 13, 957 (2006)
- 7-Bromoindirubin-3'-oxime induces caspase-independent cell death: J. Ribas, et al.; Oncogene 25, 6304 (2006)
- Indirubin, the red shade of indigo: L. Meijer, et al. (Editors): In «Life in Progress», Station Biologique, Roscoff, 297 pp. (2006)
- Indirubin derivatives inhibit malignant lymphoid cell proliferation: A. Chebel, et al.; Leuk. Lymphoma 50, 2049 (2009)
- 6-Br-5methylindirubin-3'oxime (5-Me-6-BIO) targeting the leishmanial glycogen synthase kinase-3 (GSK-3) short form affects cell-cycle progression and induces apoptosis-like death: Exploitation of GSK-3 for treating leishmaniasis: E. Xingi, et al.; Int. J. Parasit. 39, 1289 (2009)
- Anticancer effects and antimetastatic mechanisms of novel indirubin derivatives: C.A. Kressirer; Diss. Ludwig-Maximilians-Universitaet Munchen, (2010)
- 6-Bromoindirubin-3'-Oxime Inhibits JAK/STAT3 Signaling and Induces Apoptosis of Human Melanoma Cells: L. Liu, et al.; Cancer Res. 71, 3972 (2011)
