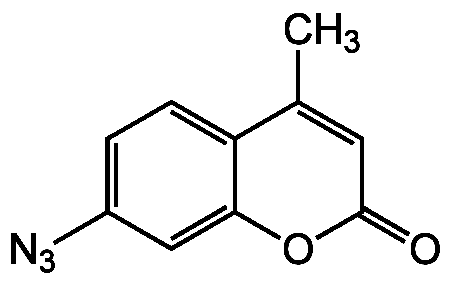
Chemical Structure
7-Azido-4-methylcoumarin [95633-27-5]

AG-CR1-3525
Overview
- SupplierAdipoGen Life Sciences
- Product Name7-Azido-4-methylcoumarin [95633-27-5]
- Delivery Days Customer10
- CAS Number95633-27-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC10H7N3O2
- Molecular Weight201.2
- Scientific DescriptionChemical. CAS: 95633-27-5. Formula: C10H7N3O2. MW: 201.2. Highly sensitive and selective fluorogenic H2S probe. The aromatic azide moiety of AzMC is selectively reduced in the presence of H2S, producing the fluorescent 7-amino-4-methylcoumarin (AMC) with a concomitant increase in fluorescence with lambdaex = 365 nm and lambdaem = 450 nm. Photoaffinity labeling probe for the substrate binding site of human sulfotransferase 1A1 (SULT1A1). Probe to monitor the enzymatic production of H2S in vitro and to visualize H2S in living cells. Tool for monitoring the activity of pyridoxal-5-phosphate (PLP)-dependent enzymes (e.g. cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CGL) and tryptophan synthase (TS)). Tool to identify novel cystathionine beta-synthase (CBS) inhibitors and activators. Suitable for high-throughput screening. Caution: Use of this product with DTT, TCEP and/or biological thiols at concentrations of >25 mM should be avoided for maximum efficiency. - Highly sensitive and selective fluorogenic H2S probe [1-3]. The aromatic azide moiety of AzMC is selectively reduced in the presence of H2S, producing the fluorescent 7-amino-4-methylcoumarin (AMC) with a concomitant increase in fluorescence with lambdaex = 365 nm and lambdaem = 450 nm. Photoaffinity labeling probe for the substrate binding site of human sulfotransferase 1A1 (SULT1A1). Probe to monitor the enzymatic production of H2S in vitro and to visualize H2S in living cells [2, 3]. Tool for monitoring the activity of pyridoxal-5-phosphate (PLP)-dependent enzymes (e.g. cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CGL) and tryptophan synthase (TS)) [2]. Tool to identify novel cystathionine beta-synthase (CBS) inhibitors and activators [2]. Suitable for high-throughput screening [2]. Caution: Use of this product with DTT, TCEP and/or biological thiols at concentrations of >25 mM should be avoided for maximum efficiency [2].
- SMILESCC1=CC(=O)OC2=CC(=CC=C12)N=[N+]=[N-]
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Photoaffinity labeling probe for the substrate binding site of human phenol sulfotransferase (SULT1A1): 7-azido-4-methylcoumarin: G. Chen, et al.; Protein Sci. 8, 2151 (1999)
- Identification of cystathionine beta-synthase inhibitors using a hydrogen sulfide selective probe: M.K. Thorson, et al.; Angew. Chem. Int. Ed. Engl. 52, 4641 (2013)
- Fluorescent probe for highly selective and sensitive detection of hydrogen sulfide in living cells and cardiac tissues: B. Chen, et al.; Analyst. 138, 946 (2013)
