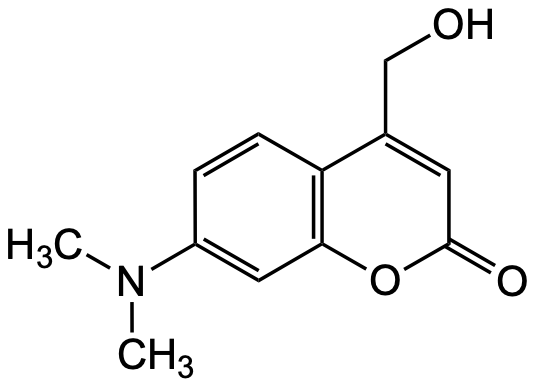
Chemical Structure
7-(Diethylamino)-4-(hydroxymethyl)coumarin [54711-38-5] [54711-38-5]
CDX-D1014
CAS Number54711-38-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight247.29
Overview
- SupplierChemodex
- Product Name7-(Diethylamino)-4-(hydroxymethyl)coumarin [54711-38-5] [54711-38-5]
- Delivery Days Customer10
- CAS Number54711-38-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC14H17NO3
- Molecular Weight247.29
- Scientific Description7-(Diethylamino)-4-(hydroxymethyl)coumarin (DEACM) is a photolabile compound that emits fluorescence upon irradiation and is used in analytical chemistry as a fluorescent probe. The photocleavable nature of DEACM facilitates its use as a photolabile protecting group for Caged bioactive molecules. The caging of bioactive molecule with photolabile protecting groups in particular has proven to be a useful tool in biochemical research. Caged compounds in which bioactive substances are inactivated with photolabile protecting groups and can be activated by UV or visible light photoirradiation. DEACM has been successfully applied for caging a wide variety of functional groups. It also has been used as a near infrared (NIR) light responsive chromophore. - Chemical. CAS: 54711-38-5. Formula: C14H17NO3. MW: 247.29. 7-(Diethylamino)-4-(hydroxymethyl)coumarin (DEACM) is a photolabile compound that emits fluorescence upon irradiation and is used in analytical chemistry as a fluorescent probe. The photocleavable nature of DEACM facilitates its use as a photolabile protecting group for Caged bioactive molecules. The caging of bioactive molecule with photolabile protecting groups in particular has proven to be a useful tool in biochemical research. Caged compounds in which bioactive substances are inactivated with photolabile protecting groups and can be activated by UV or visible light photoirradiation. DEACM has been successfully applied for caging a wide variety of functional groups. It also has been used as a near infrared (NIR) light responsive chromophore.
- SMILESCCN(CC)C1=CC2=C(C=C1)C(=CC(=O)O2)CO
- Storage InstructionRT
- UNSPSC12162000