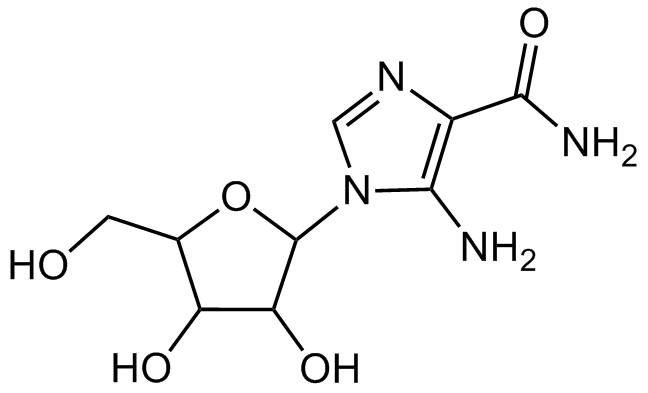
Chemical Structure
AICAR [2627-69-2]

AG-CR1-0061
Overview
- SupplierAdipoGen Life Sciences
- Product NameAICAR [2627-69-2]
- Delivery Days Customer10
- CAS Number2627-69-2
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC9H14N4O5
- Molecular Weight258.2
- Scientific DescriptionCell permeable AMP-activated protein kinase (AMPK) activator [1]. Insulin mimetic [2, 10]. Adipocyte differentiation inhibitor [3]. Apoptosis inducer [4, 11]. PPARalpha inhibitor [5]. mTOR inhibitor [6]. P70S6K inhibitor [6]. LPS-induced TNF-alpha production inhibitor [7]. TORC2 phosphorylation inducer [8]. Anti-inflammatory [9]. Anti-tumor compound [12]. Autophagy inhibitor [13]. HSP90 inhibitor [14]. Autophagy inducer [15] - Chemical. CAS: 2627-69-2. Formula: C9H14N4O5. MW: 258.2. Cell permeable AMP-activated protein kinase (AMPK) activator. Insulin mimetic. Adipocyte differentiation inhibitor. Apoptosis inducer. PPARalpha inhibitor. mTOR inhibitor. P70S6K inhibitor. LPS-induced TNF-alpha production inhibitor. TORC2 phosphorylation inducer. Anti-inflammatory. Anti-tumor compound. Autophagy inhibitor. HSP90 inhibitor. Autophagy inducer
- SMILESNC(=O)C1=C(N)N(C=N1)C1OC(CO)C(O)C1O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells?: J. M. Corton et al.; Eur. J. Biochem. 229, 558 (1995)
- 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase: P. A, Lochhead et al.; Diabetes 49, 896 (2000)
- The effects of AICAR on adipocyte differentiation of 3T3-L1 cells: S. A. Habinowski et al.; Biochem. Biophys. Res. Commun.286, 852 (2001)
- 5-Aminoimidazole-4-carboxamide riboside induces apoptosis in Jurkat cells, but the AMP-activated protein kinase is not involved: J. M. Lopez et al.; Biochem. J. 370, 1027 (2003)
- Kinase-independent transcriptional co-activation of peroxisome proliferator-activated receptor alpha by AMP-activated protein kinase: M. Bronner et al.; Biochem. J. 384, 295 (2004)
- AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms: X. Xiang et al.; Biochem. Biophys. Res. Commun. 321, 161 (2004)
- 5-Aminoimidazole-4-carboxamide riboside suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of phosphatidylinositol 3-kinase/Akt activation in RAW 264.7 murine macrophages: B. S. Jhun et al.; Biochem. Biophys. Res. Commun.318, 372 (2004)
- The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism: S. H. Koo et al.; Nature 437, 1109 (2005)
- 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase: S. Giri, et al.; J. Neurosci. 24, 479 (2004)
- 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes: I.P. Salt, et al.; Diabetes 49, 1649 (2000)
