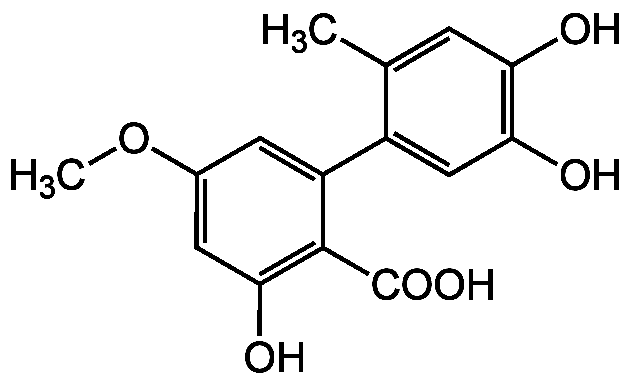
Chemical Structure
Altenusin [31186-12-6]

AG-CN2-0143
Overview
- SupplierAdipoGen Life Sciences
- Product NameAltenusin [31186-12-6]
- Delivery Days Customer10
- CAS Number31186-12-6
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationWarning
- Molecular FormulaC15H14O6
- Molecular Weight290.3
- Scientific DescriptionAntibiotic [7]. Antifungal penicillide [8]. Non-competitive, specific neutral sphingomyelinase (N-SMase) inhibitor [2]. Strong pp60c-Src inhibitor [3]. Inhibits cFMS receptor tyrosine kinase (CSF-1/m-CSF receptor tyrosine kinase) which is implicated in cancer and bone diseases [3]. Myosin light chain kinase inhibitor [1]. Exhibits anti-HIV-1 integrase activity [4]. Cytotoxic activity against mouse lymphoma cells [6]. Anti-protozoan, trypanothione reductase (TR) inhibitor [5]. Phytotoxin [9]. - Chemical. CAS: 31186-12-6. Formula: C15H14O6. MW: 290.3. Isolated from Penicillium sp. Antibiotic. Antifungal penicillide. Non-competitive, specific neutral sphingomyelinase (N-SMase) inhibitor. Strong pp60c-Src inhibitor. Inhibits cFMS receptor tyrosine kinase (CSF-1/m-CSF receptor tyrosine kinase) which is implicated in cancer and bone diseases. Myosin light chain kinase inhibitor. Exhibits anti-HIV-1 integrase activity. Cytotoxic activity against mouse lymphoma cells. Anti-protozoan, trypanothione reductase (TR) inhibitor. Phytotoxin.
- SMILESCOC1=CC(O)=C(C(O)=O)C(=C1)C1=CC(O)=C(O)C=C1C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Isolation of myosin light chain kinase inhibitors from microorganisms: dehydroaltenusin, altenusin, atrovenetinone, and cyclooctasulfur: S. Nakanishi, et al.; Biosci. Biotechnol. Biochem. 59, 1333 (1995)
- Alutenusin, a specific neutral sphingomyelinase inhibitor, produced by Penicillium sp. FO-7436: R. Uchida, et al.; J. Antibiot. 52, 572 (1999)
- Fungal Metabolites as Potent Protein Kinase Inhibitors: Identification of a Novel Metabolite and Novel Activities of Known Metabolites: M. Oyama, et al.; Lett. Drug Des. Disc. 1, 24 (2004)
- HIV-1 Integrase Inhibitors: 2003-2004 Update: R. Dayan, et al.; Med. Res. Rev. 26, 271 (2006)
- Altenusin, a biphenyl isolated from the endophytic fungus Alternaria sp. inhibits trypanothione reductase from Trypanosoma cruzi: B.B. Cota, et al.; FEMS Microbiol. Lett. 285, 177 (2008)
- Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense: A.H. Aly, et al.; J. Nat. Prod. 71, 972 (2008)
- Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp.: Q.X. Wang, et al.; Fitoterapia 83, 209 (2012)
- Antifungal activity of altenusin isolated from the endophytic fungus Alternaria sp. against the pathogenic fungus Paracoccidioides brasiliensis: S. Johann, et al.; Rev. Iberoam. Micol. 29, 205 (2012)
- Identification of Alternaria alternata mycotoxins by LC-SPE-NMR and their cytotoxic effects to soybean (Glycine max) cell suspension culture: G.D. de Souza, et al.; Molecules 18, 2528 (2013)
