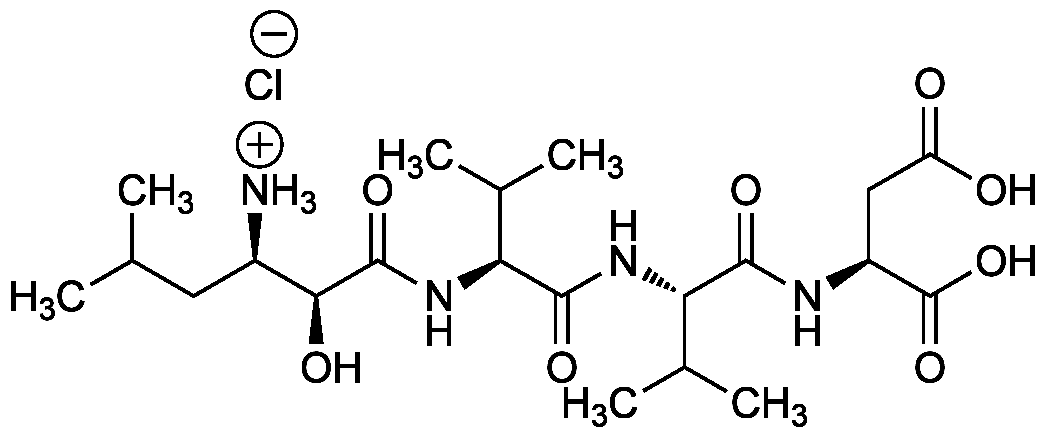
Chemical Structure
Amastatin . hydrochloride [100938-10-1] [100938-10-1]
AG-CP3-7003
CAS Number100938-10-1
Product group Chemicals
Estimated Purity>98%
Molecular Weight474.6 . 36.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameAmastatin . hydrochloride [100938-10-1] [100938-10-1]
- Delivery Days Customer10
- CAS Number100938-10-1
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC21H38N4O8 . HCl
- Molecular Weight474.6 . 36.5
- Scientific DescriptionChemical. CAS: 100938-10-1. Formula: C21H38N4O8 . HCl. MW: 474.6 . 36.5. Synthetic. Slow, tight binding and competitive aminopeptidase (AP) inhibitor. Inhibits cytosolic leucine aminopeptidase, microsomal aminopeptidase M and bacterial leucine aminopeptidase, human serum aminopeptidase A (AP-A), aminopeptidase N (AP-N), tyrosine aminopeptidase, but not aminopeptidase B (AP-B). Amastatin is without effect on trypsin, papain, chymotrypsin, elastase, pepsin or thermolysin. Inhibits completely the Suc-Ala-Ala-Pro-Leu-pNA amidolytic enzyme. Slightly inhibits the formation of angiotensin III (Ang III) from Angiotensin II through AP-A, but significantly increases the potency of angiotensin III and-angiotensin I. Moderate inhibitor of mitochondrial intermediate peptidase (MIP). Weak inhibitor of simian immunodeficiency virus protease (SIV-PR). - Slow, tight binding and competitive aminopeptidase (AP) inhibitor. Inhibits cytosolic leucine aminopeptidase, microsomal aminopeptidase M and bacterial leucine aminopeptidase, human serum aminopeptidase A (AP-A), aminopeptidase N (AP-N), tyrosine aminopeptidase, but not aminopeptidase B (AP-B). Amastatin is without effect on trypsin, papain, chymotrypsin, elastase, pepsin or thermolysin. Inhibits completely the Suc-Ala-Ala-Pro-Leu-pNA amidolytic enzyme. Slightly inhibits the formation of angiotensin III (Ang III) from Angiotensin II through AP-A, but significantly increases the potency of angiotensin III and [des-Asp1]-angiotensin I. Moderate inhibitor of mitochondrial intermediate peptidase (MIP). Weak inhibitor of simian immunodeficiency virus protease (SIV-PR). ANPEP (aminopeptidase N) is a host receptor targeted by porcine epidemic diarrhoea virus, human coronavirus 229E, feline coronavirus, canine coronavirus, transmissible gastroenteritis virus and infectious bronchitis virus. These viruses all belong to coronaviridae. ANPEP is therefore investigated as a potential target for SARS-CoV-2 infections.
- SMILES[Cl-].CC(C)C[C@@H]([NH3+])[C@H](O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Amastatin hydrochloride [100938-10-1] [100938-10-1]](https://www.targetmol.com/group3/M00/02/AA/CgoaEWY7NLCEdQIBAAAAAFwUOgY591.png)