Chemical Structure
AMCA-H [106562-32-7]
CDX-A0009
CAS Number106562-32-7
Product group Chemicals
Estimated Purity>90%
Molecular Weight233.22
Overview
- SupplierChemodex
- Product NameAMCA-H [106562-32-7]
- Delivery Days Customer2
- CAS Number106562-32-7
- CertificationResearch Use Only
- Estimated Purity>90%
- Hazard InformationWarning
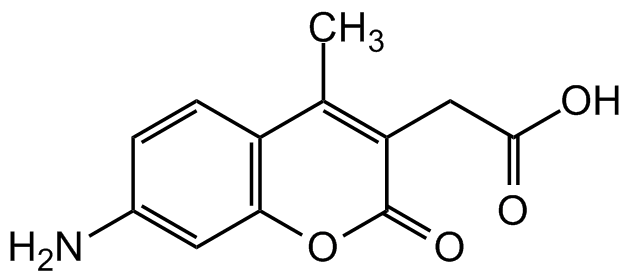
- Molecular FormulaC12H11NO4
- Molecular Weight233.22
- Scientific DescriptionAMCA is one of the brightest amine-reactive blue fluorescent dyes useful for immunofluorescence and fluorescent labeling (Ex/Em: 353/455nm). It is quite photostable, and its fluorescence is pH-independent from pH 4 to 10. The properties include a relatively large Stokes shift and resistance to photobleaching. Reactive AMCA derivatives are used as contrasting probes for double and triple labeling in immunofluorescence microscopy, arrays and in situ hybridization. NHS-AMCA and Sulfo-NHS-AMCA are reactive towards primary amine groups on antibodies, proteins, peptides and other biomolecules. AMCA-Hydrazide is used to label glycosylation sites or for conjugation to carboxyl groups using the crosslinker EDC. - Chemical. CAS: 106562-32-7. Formula: C12H11NO4. MW: 233.22. Synthetic. AMCA is one of the brightest amine-reactive blue fluorescent dyes useful for immunofluorescence and fluorescent labeling (Ex/Em: 353/455nm). It is quite photostable, and its fluorescence is pH-independent from pH 4 to 10. The properties include a relatively large Stokes shift and resistance to photobleaching. Reactive AMCA derivatives are used as contrasting probes for double and triple labeling in immunofluorescence microscopy, arrays and in situ hybridization. NHS-AMCA and Sulfo-NHS-AMCA are reactive towards primary amine groups on antibodies, proteins, peptides and other biomolecules. AMCA-Hydrazide is used to label glycosylation sites or for conjugation to carboxyl groups using the crosslinker EDC.
- SMILESCC1=C(CC(O)=O)C(=O)OC2=C1C=CC(N)=C2
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12162000