Chemical Structure
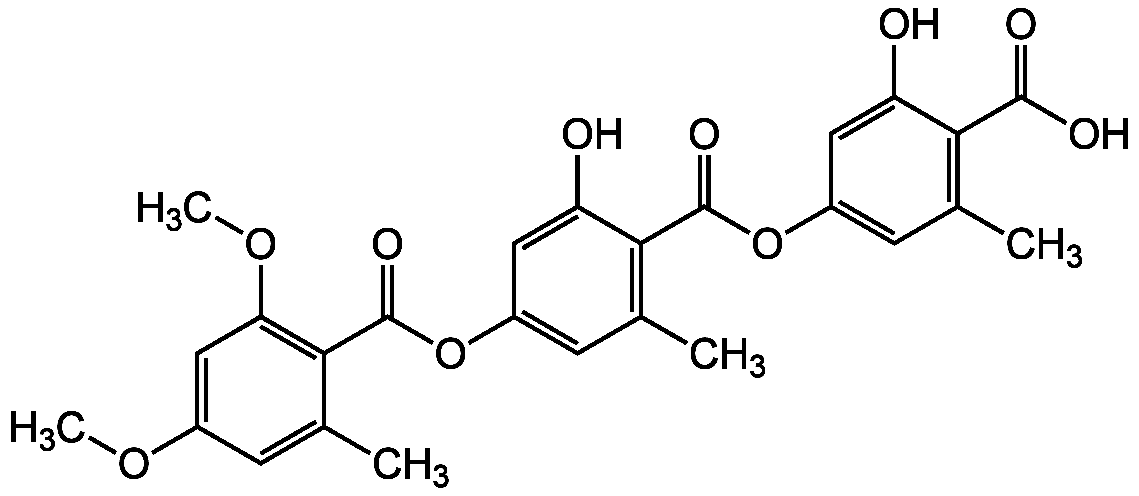
Amidepsine D [79786-34-8]

AG-CN2-0110
Overview
- SupplierAdipoGen Life Sciences
- Product NameAmidepsine D [79786-34-8]
- Delivery Days Customer10
- CAS Number79786-34-8
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC26H24O10
- Molecular Weight496.5
- Scientific DescriptionChemical. CAS: 79786-34-8. Formula: C26H24O10. MW: 496.5. Isolated from Humcola sp. FO-2942. Diacylglycerol acyltransferase (DGAT) inhibitor. Excessive accumulation of triacetylglycerol produced by DGAT may cause fatty liver, obesity and hypertriglyceridemia, which may lead to atherosclerosis, diabetes and metabolic disorders. - Diacylglycerol acyltransferase (DGAT) inhibitor [1-4]. Excessive accumulation of triacetylglycerol produced by DGAT may cause fatty liver, obesity and hypertriglyceridemia, which may lead to atherosclerosis, diabetes and metabolic disorders.
- SMILESCOC1=CC(OC)=C(C(=O)OC2=CC(C)=C(C(=O)OC3=CC(C)=C(C(O)=O)C(O)=C3)C(O)=C2)C(C)=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Amidepsines, inhibitors of diacylglycerol acyltransferase produced by Humicola sp. FO-2942. I. Production, isolation and biological properties: H. Tomoda, et al.; J. Antibiot. 48, 937 (1995)
- Amidepsines, inhibitors of diacylglycerol acyltransferase produced by Humicola sp. FO-2942. II. Structure elucidation of amidepsines A, B and C: H. Tomoda, et al.; J. Antibiot. 48, 942 (1995)
- Amidepsine E, an inhibitor of diacylglycerol acyltransferase produced by Humicola sp. FO-5969: H. Tomoda, et al.; J. Antibiot. 49, 929 (1996)
- Potential therapeutics for obesity and atherosclerosis: inhibitors of neutral lipid metabolism from microorganisms: H. Tomoda & S. Omura; Pharmacol. Ther. 115, 375 (2007)
