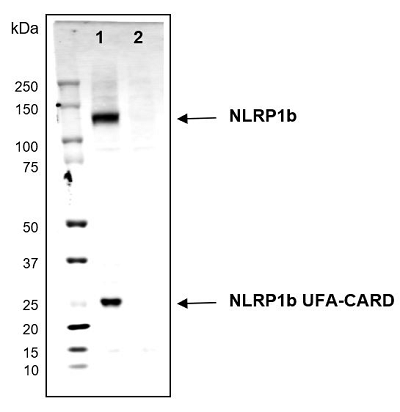
Mouse NLRP1b (mouse) (full-length and UFA-CARD fragment) is detected in Raw264.7 cells using NLRP1b, mAb (2A12) (Prod. No. AG-20B-0084). Method: Cell extracts (about 30microg) from the mouse cells Raw264.7 (lane 1) or Raw264.7 NLRP1b KO (lane 2) are separated by SDS-PAGE under reducing conditions, transferred to nitrocellulose and incubated with the NLRP1b, mAb (2A12) (1microg/ml). After incubation with anti-mouse Alexa 633 conjugated antibody, proteins are visualized by a fluorescence detection system at 700nm. Picture Courtesy of Dr Andrew Sandstrom, UT Southwestern Medical Center.
anti-NLRP1b (mouse), mAb (2A12)
AG-20B-0084
ApplicationsWestern Blot
Product group Antibodies
ReactivityMouse, Rat
Overview
- SupplierAdipoGen Life Sciences
- Product Nameanti-NLRP1b (mouse), mAb (2A12)
- Delivery Days Customer10
- ApplicationsWestern Blot
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone ID2A12
- Concentration1 mg/ml
- Estimated Purity>95%
- HostMouse
- IsotypeIgG2b
- Protein IDQ2LKW6
- Protein NameNACHT, LRR and PYD domains-containing protein 1b allele 1
- Scientific DescriptionMonoclonal Antibody. Recognizes mouse and rat NLRP1b. Detects mouse NLRP1b and also both the B6 and 129 alleles of murine NLRP1b. Does not cross-react with human NLRP1. Applications: WB. Clone: 2A12. Isotype: Mouse IgG2b. AK10383 The NLRP1 inflammasome is a multiprotein complex that is a potent activator of inflammation. As inflammasome-forming sensor protein, NLRP1b, upon detection of microbial molecules or pathogen-encoded activities, serves as a platform for the recruitment and activation of proinflammatory caspases including caspase-1 through a caspase activation and recruitment domain (CARD). Active caspase-1 mediates the maturation and release of the proinflammatory cytokines interleukin IL-1beta and IL-18. Mouse NLRP1b can be activated through proteolytic cleavage by the bacterial Lethal Toxin (LeTx) protease, resulting in degradation of the N-terminal domains of NLRP1b and liberation of the bioactive C-terminal domain, which includes the caspase activation and recruitment domain (CARD). NLRP1b has an unusual domain architecture, containing a CARD at its C-terminus rather than the N-terminus like all other inflammasomes, and a function-to-find domain (FIIND), which is located between the LRRs and CARD. The FIIND undergoes a constitutive self-cleavage event, such that NLRP1b exists in its non-activated state as two, noncovalently associated polypeptides, the N-terminal domains and the C-terminal CARD-containing fragment. Inflammasome activation is the result of site-specific cleavage in the N-terminus of mouse NLRP1b by the Lethal Factor (LF) protease subunit of LeTx, which results in its activation. Upon cleavage by LF, a novel N-terminus is formed, which is then targeted for proteasomal degradation by a protein quality control mechanism called the N-end rule pathway. Since the proteasome is a processive protease, it progressively degrades the N-terminal domains of NLRP1b but is disengaged upon arriving at the self-cleavage site within the FIIND domain. Degradation of the N-terminal domains thus releases the bioactive C-terminal CARD-containing fragment of NLRP1b, which is sufficient to initiate downstream inflammasome activation. Genetic variations in the NLRP1/NALP1 gene are associated with susceptibility to vitiligo-associated multiple autoimmune disease type 1, an autoimmune disorder characterized by the association of vitiligo with several autoimmune and autoinflammatory diseases including autoimmune thyroid disease, rheumatoid arthritis and systemic lupus erythematosus. - The NLRP1 inflammasome is a multiprotein complex that is a potent activator of inflammation. As inflammasome-forming sensor protein, NLRP1b, upon detection of microbial molecules or pathogen-encoded activities, serves as a platform for the recruitment and activation of proinflammatory caspases including caspase-1 through a caspase activation and recruitment domain (CARD). Active caspase-1 mediates the maturation and release of the proinflammatory cytokines interleukin IL-1beta and IL-18. Mouse NLRP1b can be activated through proteolytic cleavage by the bacterial Lethal Toxin (LeTx) protease, resulting in degradation of the N-terminal domains of NLRP1b and liberation of the bioactive C-terminal domain, which includes the caspase activation and recruitment domain (CARD). NLRP1b has an unusual domain architecture, containing a CARD at its C-terminus rather than the N-terminus like all other inflammasomes, and a function-to-find domain (FIIND), which is located between the LRRs and CARD. The FIIND undergoes a constitutive self-cleavage event, such that NLRP1b exists in its non-activated state as two, noncovalently associated polypeptides, the N-terminal domains and the C-terminal CARD-containing fragment. Inflammasome activation is the result of site-specific cleavage in the N-terminus of mouse NLRP1b by the Lethal Factor (LF) protease subunit of LeTx, which results in its activation. Upon cleavage by LF, a novel N-terminus is formed, which is then targeted for proteasomal degradation by a protein quality control mechanism called the N-end rule pathway. Since the proteasome is a processive protease, it progressively degrades the N-terminal domains of NLRP1b but is disengaged upon arriving at the self-cleavage site within the FIIND domain. Degradation of the N-terminal domains thus releases the bioactive C-terminal CARD-containing fragment of NLRP1b, which is sufficient to initiate downstream inflammasome activation. Genetic variations in the NLRP1/NALP1 gene are associated with susceptibility to vitiligo-associated multiple autoimmune disease type 1, an autoimmune disorder characterized by the association of vitiligo with several autoimmune and autoinflammatory diseases including autoimmune thyroid disease, rheumatoid arthritis and systemic lupus erythematosus.
- ReactivityMouse, Rat
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116161

