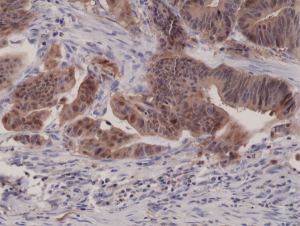
Immunohistochemical staining of formalin fixed and paraffin embedded human colon cancer tissue sections using Anti-p16INK4a RM267 at a 1:1000 dilution.
anti-p16INK4a (human), Rabbit Monoclonal (RM267)
REV-31-1144-00
ApplicationsWestern Blot, ImmunoHistoChemistry
Product group Antibodies
ReactivityHuman
TargetCDKN2A
Overview
- SupplierRevMAb Biosciences
- Product Nameanti-p16INK4a (human), Rabbit Monoclonal (RM267)
- Delivery Days Customer2
- ApplicationsWestern Blot, ImmunoHistoChemistry
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDRM267
- Gene ID1029
- Target nameCDKN2A
- Target descriptioncyclin dependent kinase inhibitor 2A
- Target synonymsARF, CAI2, CDK4I, CDKN2, CMM2, INK4, INK4A, MLM, MTS-1, MTS1, P14, P14ARF, P16, P16-INK4A, P16INK4, P16INK4A, P19, P19ARF, TP16, cyclin-dependent kinase inhibitor 2A, CDK4 inhibitor p16-INK4, CDKN2A/ARF Intron 2 lncRNA, alternative reading frame, cell cycle negative regulator beta, cyclin-dependent kinase 4 inhibitor A, cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4), inhibitor of cdk4 A, multiple tumor suppressor 1, multiple tumour suppressor 1
- HostRabbit
- IsotypeIgG
- Protein IDP42771
- Protein NameCyclin-dependent kinase inhibitor 2A
- Scientific DescriptionRecombinant Antibody. This antibody reacts to human p16INK4a (Cyclin-dependent kinase inhibitor 2A). Applications: WB, IHC. Source: Rabbit. Liquid. 50% Glycerol/PBS with 1% BSA and 0.09% sodium azide. Under normal circumstances, cell cycle initiation and progression is cooperatively regulated by several classes of cyclin-dependent kinases (CDKs), whose activities are in turn regulated by CDK inhibitors (CDKIs). To allow cell cycle progression, retinoblastoma protein (pRB) is phosphorylated by a holoenzyme complex containing cyclin D and a cyclin-dependent kinase (CDK4 or CDK6). The INK4 family of CDKIs comprises a number of small proteins, including p16INK4a, p14ARF (murine p19ARF), p15INK4b and p18INK4c, all of which have a role in cell cycle regulation. p16INK4a has long been recognized as mediator of senescence. p16INK4a binds and induces an allosteric conformational change in CDK4/CDK6 that inhibits the binding of ATP and substantially reduces the formation of the CDK4/6-cyclin D interface, which leads to the disruption of the interaction with D-type cyclins. This antagonizes cyclin binding and activation of CDK, thereby maintaining pRB in its hypophosphorylated and growth-suppressive state and induces G1 cell cycle arrest. Rb exists predominantly in an under-phosphorylated form that acts as a negative regulator of the cell cycle by sequestering transcription factors such as E2F. In the hyperphosphorylated state, Rb releases these factors, enabling them to activate transcription and drive the cell into S phase. p16INK4a plays an important role in aging. The accumulation of persistent and increasing DNA damage in senescent cells in response to telomere shortening, DNA damage, inappropriate activation of signaling pathways and production of ROS during aging results in transcriptional activation of the INK4a/ARF locus. Mitogen stimulation may amplify the signals of DNA damage. Upregulation of p16INK4a and ARF activates pRB and p53 pathways, which in turn lead to cell cycle arrest and regenerative defects. The p16INK4a/CDK/cyclin/Rb/E2F regulatory cycle exhibits exquisite control on cell growth and any alterations that upset this balance may lead to a deregulation of cell growth. Perhaps for this reason, alterations at every component of this pathway have been identified in tumor cells and include gene amplification, mutation and deletion, inhibition of transcription by DNA hypermethylation or mRNA stability, and changes in the rate of protein degradation. Deletion is the most common mechanism of p16INK4a gene inactivation, having been observed in a wide variety of tumor-derived cell lines and primary tumors. - Under normal circumstances, cell cycle initiation and progression is cooperatively regulated by several classes of cyclin-dependent kinases (CDKs), whose activities are in turn regulated by CDK inhibitors (CDKIs). To allow cell cycle progression, retinoblastoma protein (pRB) is phosphorylated by a holoenzyme complex containing cyclin D and a cyclin-dependent kinase (CDK4 or CDK6). The INK4 family of CDKIs comprises a number of small proteins, including p16INK4a, p14ARF (murine p19ARF), p15INK4b and p18INK4c, all of which have a role in cell cycle regulation. p16INK4a has long been recognized as mediator of senescence. p16INK4a binds and induces an allosteric conformational change in CDK4/CDK6 that inhibits the binding of ATP and substantially reduces the formation of the CDK4/6-cyclin D interface, which leads to the disruption of the interaction with D-type cyclins. This antagonizes cyclin binding and activation of CDK, thereby maintaining pRB in its hypophosphorylated and growth-suppressive state and induces G1 cell cycle arrest. Rb exists predominantly in an under-phosphorylated form that acts as a negative regulator of the cell cycle by sequestering transcription factors such as E2F. In the hyperphosphorylated state, Rb releases these factors, enabling them to activate transcription and drive the cell into S phase. p16INK4a plays an important role in aging. The accumulation of persistent and increasing DNA damage in senescent cells in response to telomere shortening, DNA damage, inappropriate activation of signaling pathways and production of ROS during aging results in transcriptional activation of the INK4a/ARF locus. Mitogen stimulation may amplify the signals of DNA damage. Upregulation of p16INK4a and ARF activates pRB and p53 pathways, which in turn lead to cell cycle arrest and regenerative defects. The p16INK4a/CDK/cyclin/Rb/E2F regulatory cycle exhibits exquisite control on cell growth and any alterations that upset this balance may lead to a deregulation of cell growth. Perhaps for this reason, alterations at every component of this pathway have been identified in tumor cells and include gene amplification, mutation and deletion, inhibition of transcription by DNA hypermethylation or mRNA stability, and changes in the rate of protein degradation. Deletion is the most common mechanism of p16INK4a gene inactivation, having been observed in a wide variety of tumor-derived cell lines and primary tumors.
- ReactivityHuman
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116161







