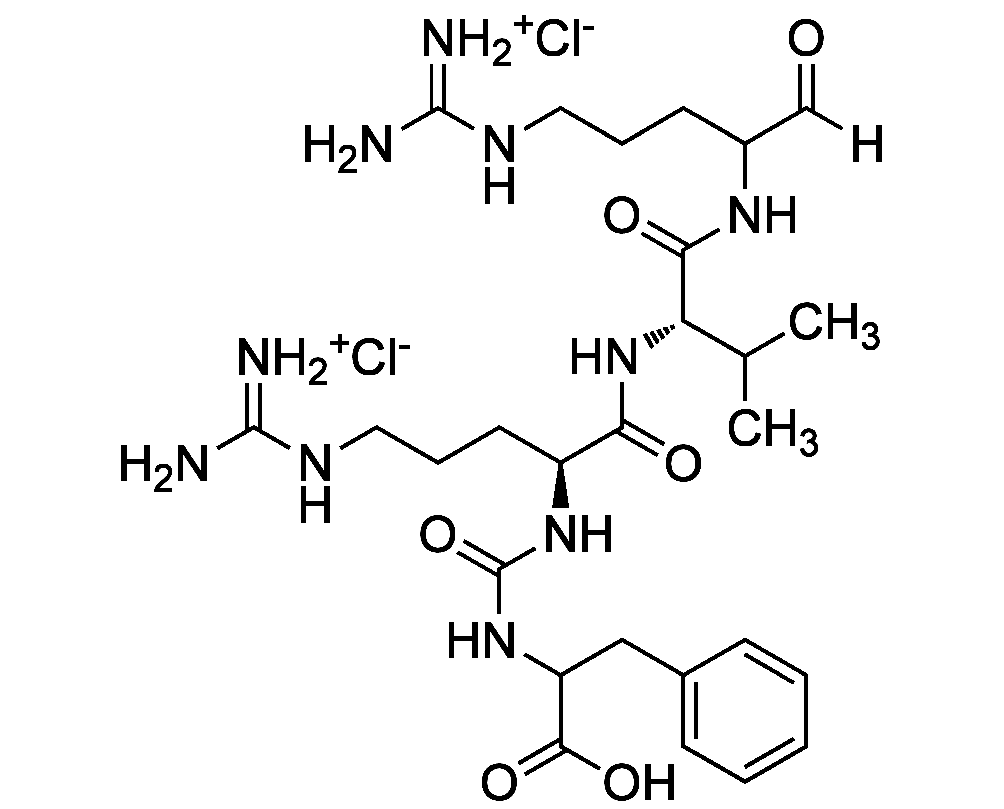
Chemical Structure
Antipain . dihydrochloride [37682-72-7]

AG-CP3-7002
CAS Number37682-72-7
Product group Chemicals
Estimated Purity>97%
Molecular Weight604.7 . 72.9
Overview
- SupplierAdipoGen Life Sciences
- Product NameAntipain . dihydrochloride [37682-72-7]
- Delivery Days Customer10
- CAS Number37682-72-7
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC27H44N10O6 . 2HCl
- Molecular Weight604.7 . 72.9
- Scientific DescriptionChemical. CAS: 37682-72-7. Formula: C27H44N10O6 . 2HCl. MW: 604.7 . 72.9. Synthetic. Potent reversible inhibitor of serine/cysteine proteases and some trypsin-like proteases. Inhibits papain, trypsin and trypsin-like serine proteases as well as cathepsin A, B and D. Similar activity spectrum comparable to leupeptin (AG-CP3-7000). Acts by forming a hemiacetal adduct between its aldehyde group and the active serine of the proteinase. Inhibits transformation of NIH3T3 cells after transfection with an activated H-ras oncogene. Used to evaluate the role of proteases in the process of malignant cell transformations. Shows antibacterial and antiparasitic activity. - Potent reversible inhibitor of serine/cysteine proteases and some trypsin-like proteases. Inhibits papain, trypsin and trypsin-like serine proteases as well as cathepsin A, B and D. Similar activity spectrum comparable to leupeptin (AG-CP3-7000). Acts by forming a hemiacetal adduct between its aldehyde group and the active serine of the proteinase. Inhibits transformation of NIH3T3 cells after transfection with an activated H-ras oncogene. Used to evaluate the role of proteases in the process of malignant cell transformations. Shows antibacterial and antiparasitic activity.
- SMILES[Cl-].[Cl-].[H]C(=O)C(CCCNC(N)=[NH2+])NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)NC(CC1=CC=CC=C1)C(O)=O)C(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Antipain, a new protease inhibitor isolated from actinomycetes: H. Suda, et al.; J. Antibiot. 25, 263 (1972)
- Structures and activities of protease inhibitors of microbial origin: H. Umezawa; Methods Enzymol. 45, 678 (1976)
- Inhibition of proteolytic activity of calcium activated neutral protease by leupeptin and antipain: T. Toyo-Oka, et al.; BBRC 82, 484 (1978)
- Effects of antipain (a protease inhibitor) on respiration, viability, and excision of pyrimidine dimers in UV-irradiated Escherichia coli cells: P.A. Swenson & R.L. Schenley; J. Bacteriol. 135, 1167 (1978)
- Effect of Ca2+ on the inhibition of calcium-activated neutral protease by leupeptin, antipain and epoxysuccinate derivatives: K. Suzuki, et al.; FEBS Lett. 136, 119 (1981)
- Inhibition of Leishmania amastigote growth by antipain and leupeptin: G.H. Coombs & J. Baxter; Ann. Trop. Med. Parasitol. 78, 21 (1984)
- Antipain or leupeptin in combination with aspirin or indomethacin synergistically inhibit human platelet activation by thrombin and trypsin: M. Ruggiero & E.G. Lapetina; Adv. Prostaglandin Thromboxane Leukot. Res. 17A, 563 (1987)
- Inhibition of H-ras oncogene transformation of NIH3T3 cells by protease inhibitors: S.J. Garte, et al.; Cancer Res. 47, 3159 (1987)
- Reversible conversion of tyrosine hydroxylase to an inactive form by antipain: S. Okuno & H. Fujisawa; BBRC 146, 1375 (1987)
- Antipain-induced suppression of oncogene expression in H-ras-transformed NIH3T3 cells: L.R. Cox, et al.; Cancer Res. 51, 4810 (1991)
