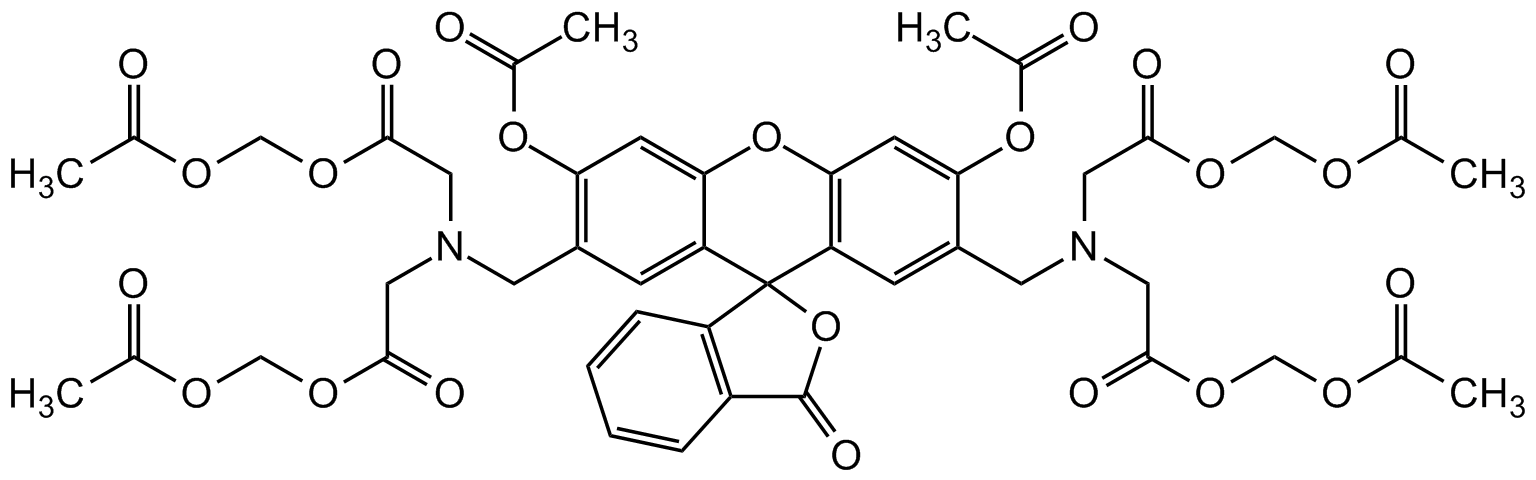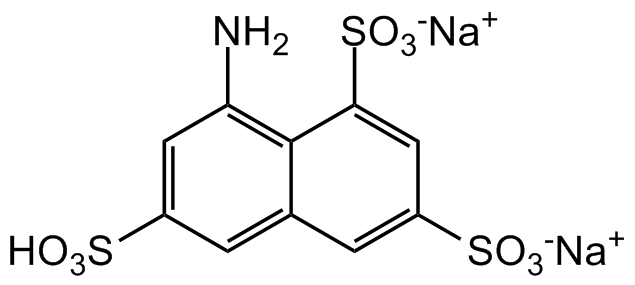
Chemical Structure
ANTS [5398-34-5] [5398-34-5]
CDX-A0071
CAS Number5398-34-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight427.34
Overview
- SupplierChemodex
- Product NameANTS [5398-34-5] [5398-34-5]
- Delivery Days Customer10
- CAS Number5398-34-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC10H7NNa2O9S3
- Molecular Weight427.34
- Scientific DescriptionANTS is a highly negatively charged dye with an amino group that can be coupled to an aldehyde or ketone group to form an unstable Schiff base. The Schiff base is usually chemically reduced by sodium borohydride (NaBH4) or sodium cyanoborohydride (NaB(CN)H3) to form a stable linkage. This labeling technique has been widely used for the labeling and subsequent sequencing of oligosaccharides and glycoproteins. The negative charges of the dye facilitate the electrophoretic separation of the degradation products of carbohydrate polymers. The polyanionic dye ANTS is used together with the fluorescent cationic quencher DPX for membrane fusion studies or permeability assays. The mixture of ANTS-DPX is minimally fluorescent initially, but becomes increasingly more fluorescent upon dilution (i.e. membrane fusion or leakage). ANTS also is used as a neuronal tracer. ANTS has a relatively high Stokes shift in water, which sufficiently separates its emission from the majority of the autofluorescence of biological samples. Spectral Data: lambdaex 356 nm; lambdaem 512 nm in 0.1 M phosphate pH 7.0. - Chemical. CAS: 5398-34-5. Formula: C10H7NNa2O9S3. Molecular Weight: 427.34. ANTS is a highly negatively charged dye with an amino group that can be coupled to an aldehyde or ketone group to form an unstable Schiff base. The Schiff base is usually chemically reduced by sodium borohydride (NaBH4) or sodium cyanoborohydride (NaB(CN)H3) to form a stable linkage. This labeling technique has been widely used for the labeling and subsequent sequencing of oligosaccharides and glycoproteins. The negative charges of the dye facilitate the electrophoretic separation of the degradation products of carbohydrate polymers. The polyanionic dye ANTS is used together with the fluorescent cationic quencher DPX for membrane fusion studies or permeability assays. The mixture of ANTS-DPX is minimally fluorescent initially, but becomes increasingly more fluorescent upon dilution (i.e. membrane fusion or leakage). ANTS also is used as a neuronal tracer. ANTS has a relatively high Stokes shift in water, which sufficiently separates its emission from the majority of the autofluorescence of biological samples. Spectral Data: lambdaex 356 nm; lambdaem 512 nm in 0.1 M phosphate pH 7.0.
- SMILESNC1=C2C(C=C(S(=O)([O-])=O)C=C2S(=O)([O-])=O)=CC(S(=O)(O)=O)=C1.[Na+].[Na+]
- Storage InstructionRT
- UNSPSC12162000