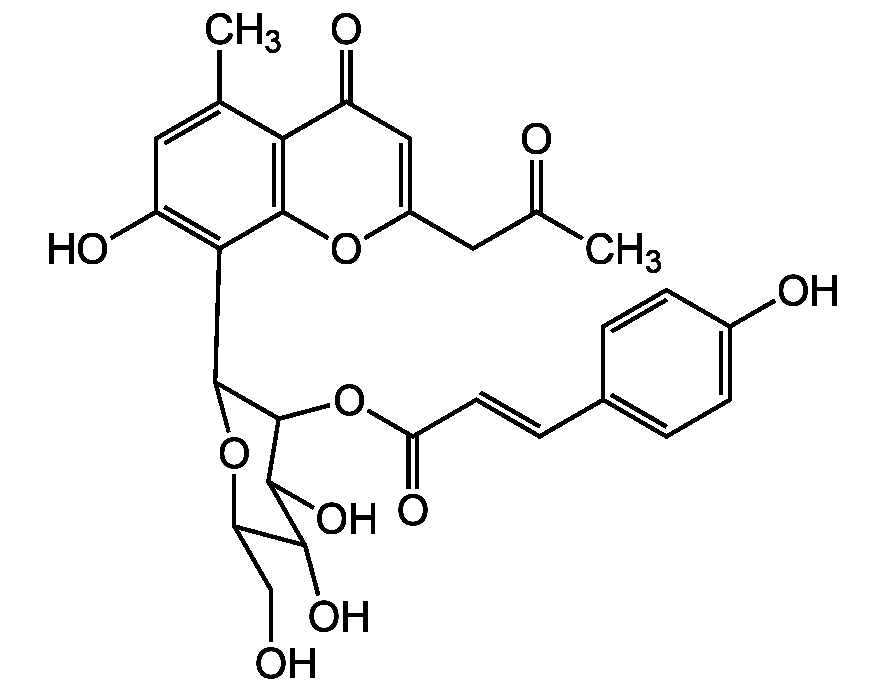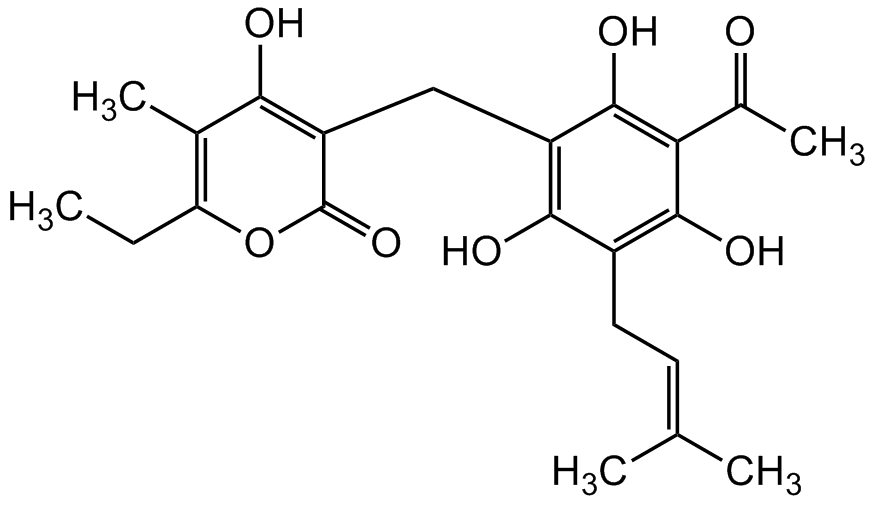
Chemical Structure
Arzanol [32274-52-5] [32274-52-5]
AG-CN2-0500
CAS Number32274-52-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight402.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameArzanol [32274-52-5] [32274-52-5]
- Delivery Days Customer10
- CAS Number32274-52-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC22H26O7
- Molecular Weight402.4
- Scientific DescriptionBrain glycogen phosphorylase (bGP) agonist. Arzanol directly interacts with bGP, competing for the same allosteric binding site on bGP like AMP (but with higher affinity), inducing similar conformational changes and promoting the transition to the active form, consequently leading to increased bGP enzyme activity. GPs are key enzymes in glycogen metabolism, promoting the rate-limiting step of its mobilization. In brain, glycogen acts as an emergency glucose storage to protect neurons against hypoglycemia and hypoxic stress, being critical for high cognitive processes such as learning and memory consolidation. Reduced glycogen breakdown is associated with impaired cognitive functions and neuro-degeneration. Activation of glycogen breakdown might be a new therapeutic strategy. Anti-inflammatory compound. Potent dual inhibitor of pro-inflammatory transcription factors and inflammatory enzymes. Inhibitor of NFkappaB activation. Potent inhibitor of inducible microsomal prostaglandin E2 synthase-1 (mPGES-1), the terminal synthase responsible for the synthesis of the pro-tumorigenic prostaglandin E2 (PGE2). Antioxidant. 5-Lipoxygenase (5-LO) inhibitor. Antibacterial compound. HIV-1 replication inhibitor. - Chemical. CAS: 32274-52-5. Formula: C22H26O7. MW: 402.4. Isolated from Helichrysum italicum. Anti-inflammatory compound. Potent dual inhibitor of pro-inflammatory transcription factors and inflammatory enzymes. Inhibitor of NFkappaB activation. Potent inhibitor of inducible microsomal prostaglandin E2 synthase-1 (mPGES-1), the terminal synthase responsible for the synthesis of the pro-tumorigenic prostaglandin E2 (PGE2). Antioxidant. 5-Lipoxygenase (5-LO) inhibitor. Antibacterial compound. HIV-1 replication inhibitor.
- SMILESCC1=C(CC)OC(C(CC2=C(O)C(C/C=C(C)/C)=C(O)C(C(C)=O)=C2O)=C1O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200