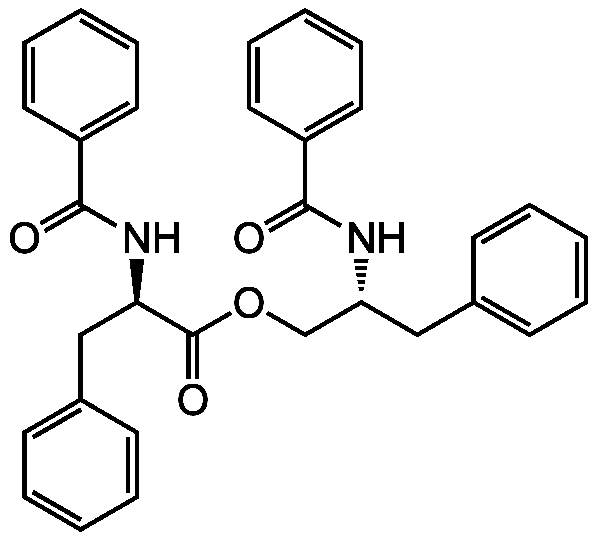
Chemical Structure
Asperphenamate [63631-36-7]

AG-CN2-0171
Overview
- SupplierAdipoGen Life Sciences
- Product NameAsperphenamate [63631-36-7]
- Delivery Days Customer10
- CAS Number63631-36-7
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC32H30N2O4
- Molecular Weight506.6
- Scientific DescriptionAnticancer compound. Cytotoxic against human breast cancer cells. Induces autophagic cell death in MCF-7 cells. Moderate radical scavenger. Weak acetylcholinesterase (AChE) inhibitor. Shows moderate trypanocidal activity. - Chemical. CAS: 63631-36-7. Formula: C32H30N2O4. MW: 506.6. Isolated from Aspergillus sp. Anticancer compound. Cytotoxic against human breast cancer cells. Induces autophagic cell death in MCF-7 cells. Moderate radical scavenger. Weak acetylcholinesterase (AChE) inhibitor. Shows moderate trypanocidal activity.
- SMILESO=C(OC[C@@H](CC1=CC=CC=C1)NC(=O)C1=CC=CC=C1)[C@@H](CC1=CC=CC=C1)NC(=O)C1=CC=CC=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Two metabolites from Aspergillus flavipes: A.M. Clark, et al.; Lloyda 40, 146 (1977)
- Cytotoxic and anti-HIV principles from the rhizomes of Begonia nantoensis: P.W. Wu, et al.; Chem. Pharm. Bull. 52, 345 (2004)
- A new method for asperphenamate synthesis and its antimicrobial activity evaluation: A.M. Pomini, et al.; Nat. Prod. Res. 20, 537 (2006)
- Total synthesis and anticancer activity studies of the stereoisomers of asperphenamate and patriscabratine: L. Yuane, et al.; Chin. Chem. Lett. 21, 155 (2010)
- JNK-dependent Atg4 upregulation mediates asperphenamate derivative BBP-induced autophagy in MCF-7 cells: Y. Li, et al.; Toxicol. Appl. Pharmacol. 263, 21 (2012)
- Two new Penicillium species Penicillium buchwaldii and Penicillium spathulatum, producing the anticancer compound asperphenamate: J.C. Frisvad, et al.; FEMS Microbiol. Lett. 339, 77 (2013)
- In vitro acetylcholinesterase activity of peptide derivatives isolated from two species of Leguminosae: C.Q. Alves, et al.; Pharm. Biol. 51, 936 (2013)
- Antioxidant activity of compounds isolated from the root woods of Erythrina droogmansiana: A.J.G. Yaya, et al.; Int. J. Pharm. Sci. Drug Res. 6, 160 (2014)
- Two trypanocidal dipeptides from the roots of Zapoteca portoricensis (Fabaceae): N.J. Nwodo, et al.; Molecules 19, 5470 (2014)
