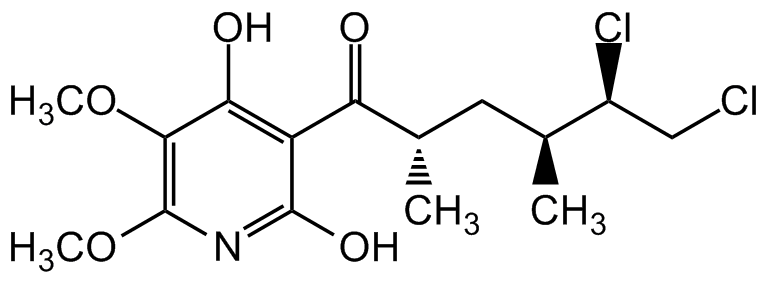
Chemical Structure
Atpenin A5
AG-CN2-0100
CAS Number119509-24-9
Product group Chemicals
Estimated Purity>95%
Molecular Weight366.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameAtpenin A5
- Delivery Days Customer10
- CAS Number119509-24-9
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC15H21Cl2NO5
- Molecular Weight366.2
- Scientific DescriptionAntibiotic [1-3]. Antifungal [1-3]. Potent and specific mitochondrial complex II (succinate-ubiquinone oxidoreductase) inhibitor [4, 5, 7]. Mitochondrial ATP-sensitive potassium (mK(ATP)) channel activator [6, 8]. Cardioprotective [6, 8]. Modulates mitochondrial ROS generation during cardioprotection [9]. - Chemical. CAS: 119509-24-9. Formula: C15H21Cl2NO5. MW: 366.2. Synthetic. Originally isolated from Penicillium sp. strain FO-125. Antibiotic. Antifungal. Potent and specific mitochondrial complex II (succinate-ubiquinone oxidoreductase) inhibitor. Mitochondrial ATP-sensitive potassium (mK(ATP)) channel activator. Cardioprotective. Modulates mitochondrial ROS generation during cardioprotection.
- SMILESCOC1=C(OC)C(O)=C(C(=O)[C@@H](C)C[C@H](C)[C@@H](Cl)CCl)C(O)=N1
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 3462
- UNSPSC12352200

![Atpenin A5 [119509-24-9]](https://www.targetmol.com/group3/M00/35/81/CgoaEGayIZ2ECMA9AAAAAFc0NTw320.png)