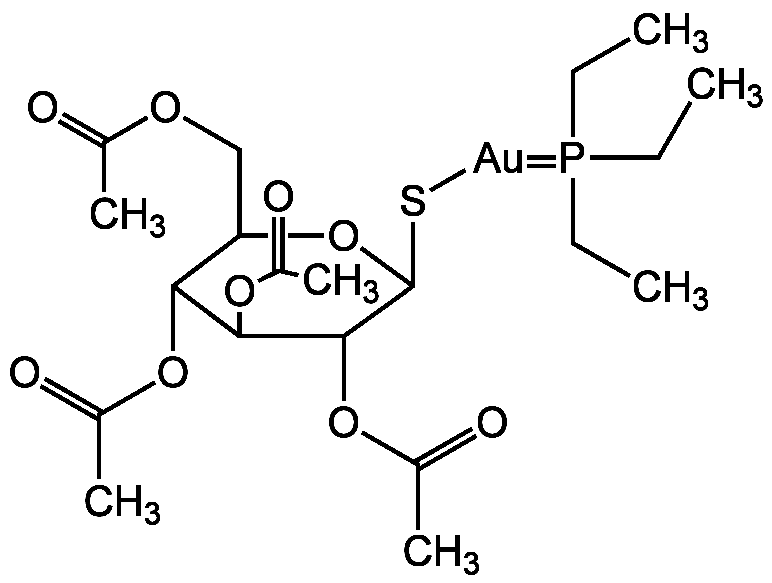
Chemical Structure
Auranofin [34031-32-8] [34031-32-8]
AG-CR1-3611
CAS Number34031-32-8
Product group Chemicals
Estimated Purity>95%
Molecular Weight678.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameAuranofin [34031-32-8] [34031-32-8]
- Delivery Days Customer10
- CAS Number34031-32-8
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC20H34AuO9PS
- Molecular Weight678.5
- Scientific DescriptionAnti-inflammatory and anti-arthritic agent. Represses the activation of the NLRP3/IL-1beta pathway. Inhibitor of 5-lipoxygenase in human neutrophils, IKB kinase (IKK) by modifying Cys-179 of the IKKbeta subunit 5. Potent thioredoxin reductase (TrxR) inhibitor. Induces mitochondrial membrane permeability transition. Anti-cancer compound with anti-proliferative activity. Shown to induce apoptosis and necrosis. STAT3 and telomerase activity inhibitor. Proteasomal deubiquitinase inhibitor. Antibiotic, antiviral and antiparasitic compound. Immunosuppressive. - Chemical. CAS: 34031-32-8. Formula: C20H34AuO9PS. MW: 678.5. Anti-inflammatory and anti-arthritic agent. Represses the activation of the NLRP3/IL-1beta pathway. Inhibitor of 5-lipoxygenase in human neutrophils, IKB kinase (IKK) by modifying Cys-179 of the IKKbeta subunit 5. Potent thioredoxin reductase (TrxR) inhibitor. Induces mitochondrial membrane permeability transition. Anti-cancer compound with anti-proliferative activity. Shown to induce apoptosis and necrosis. STAT3 and telomerase activity inhibitor. Proteasomal deubiquitinase inhibitor. Antibiotic, antiviral and antiparasitic compound. Immunosuppressive.
- SMILESCCP(CC)(CC)=[Au]SC1OC(COC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200



![Auranofin [34031-32-8] [34031-32-8]](https://www.targetmol.com/group3/M00/02/73/CgoaEGY7M8qEG7CqAAAAAFKu5LY930.png)