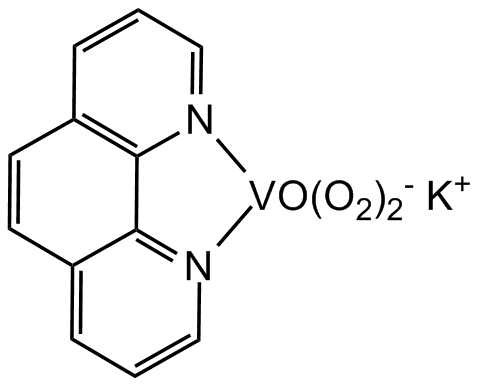
Chemical Structure
bpV(phen) [171202-16-7]
AG-CR1-0042
CAS Number171202-16-7
Product group Chemicals
Estimated Purity>99%
Molecular Weight350.2 . 54.0
Overview
- SupplierAdipoGen Life Sciences
- Product NamebpV(phen) [171202-16-7]
- Delivery Days Customer10
- CAS Number171202-16-7
- CertificationResearch Use Only
- Estimated Purity>99%
- Molecular FormulaK[VO(O2)2C12H8N2] . 3H2O
- Molecular Weight350.2 . 54.0
- Scientific DescriptionChemical. CAS: 171202-16-7. Formula: K[VO(O2)2C12H8N2] . 3H2O. MW: 350.2 . 54.0. Potent protein phosphotyrosine phosphatase inhibitor. Insulin receptor kinase (IRK) inducer. Insulin mimetic in vitro and in vivo. Apoptosis inducer. ERK inducer. Mitogen-activated protein kinase phosphatase-1 (MKP-1) inducer. Angiogenesis inhibitor. PTEN inhibitor. - Potent protein phosphotyrosine phosphatase inhibitor [1, 2, 5]. Insulin receptor kinase (IRK) inducer [2]. Insulin mimetic in vitro and in vivo [3, 4]. Apoptosis inducer [6]. ERK inducer [7]. Mitogen-activated protein kinase phosphatase-1 (MKP-1) inducer [7]. Angiogenesis inhibitor [8]. PTEN inhibitor [9].
- SMILESC1[N]2=CC=CC3=C2C2=[N]1C=CC=C2C=C3
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200