Chemical Structure
Calphostin C [121263-19-2]

AG-CN2-0430
Overview
- SupplierAdipoGen Life Sciences
- Product NameCalphostin C [121263-19-2]
- Delivery Days Customer10
- CAS Number121263-19-2
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC44H38O14
- Molecular Weight790.8
- Scientific DescriptionChemical. CAS: 121263-19-2. Formula: C44H38O14. MW: 790.8. Isolated from Cladosporium cladosporioides sp. Potent and highly specific cell permeable protein kinase C (PKC) inhibitor. The inhibition of PKC is light-dependent. PKA, PKG, DAG kinase, phospholipase D1 and D2, myosin light chain kinase and c-Src inhibitor. Anticancer compound. Inhibits cell proliferation and strongly induces apoptosis in vitro. Inducer of endoplasmic reticulum ER-stress. Shown to directly and potently block L-type Ca channels. Calphostin C specifically inhibits contraction-stimulated glucose transport but not insulin-stimulated glucose transport in skeletal muscle. Wnt/beta-catenin/lef-1 signaling inhibitor. beta-catenin/TCF antagonist. Inhibits Wnt-activated genes in a dose-dependent fashion - Potent and highly specific cell permeable protein kinase C (PKC) inhibitor [1]. The inhibition of PKC is light-dependent. PKA, PKG, DAG kinase, phospholipase D1 and D2, myosin light chain kinase and c-Src inhibitor. Anticancer compound. Inhibits cell proliferation and strongly induces apoptosis in vitro. Inducer of endoplasmic reticulum ER-stress. Shown to directly and potently block L-type Ca channels. Calphostin C specifically inhibits contraction-stimulated glucose transport but not insulin-stimulated glucose transport in skeletal muscle. Wnt/beta-catenin/lef-1 signaling inhibitor. beta-catenin/TCF antagonist. Inhibits Wnt-activated genes in a dose-dependent fashion
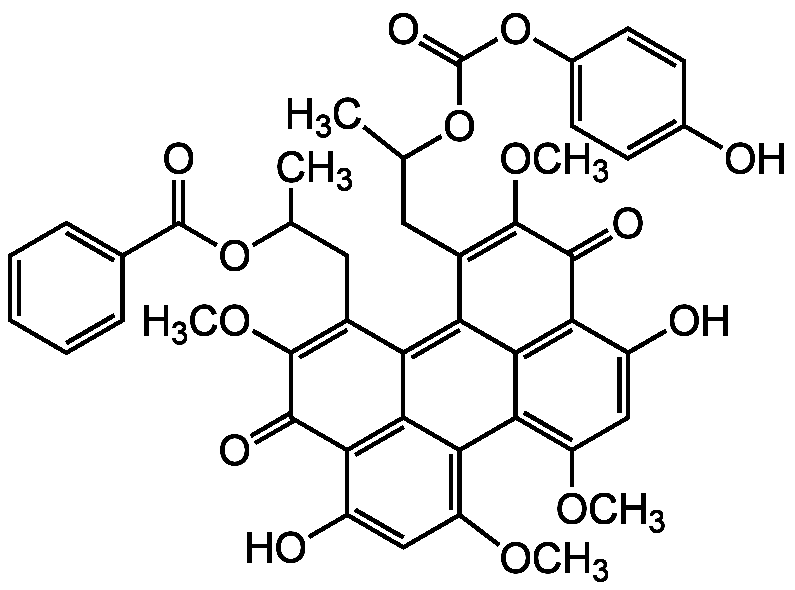
- SMILESCOC1=C2C3=C(OC)C=C(O)C4=C3C(C(CC(C)OC(=O)C3=CC=CC=C3)=C(OC)C4=O)=C3C(CC(C)OC(=O)OC4=CC=C(O)C=C4)=C(OC)C(=O)C(C(O)=C1)=C23
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Calphostins (UCN-1028), novel and specific inhibitors of protein kinase C. I. Fermentation, isolation, physico-chemical properties and biological activities: E. Kobayashi, et al.; J. Antibiot. (Tokyo) 42, 1470 (1989)
- Calphostins, novel and specific inhibitors of protein kinase C. II. Chemical structures: T. Iida, et al.; J. Antibiot. (Tokyo). 42, 1475 (1989)
- Use and specificity of staurosporine, UCN-01, and calphostin C as protein kinase inhibitors: T. Tamaoki, et al.; Methods Enzymol. 201, 340 (1991)
- Inhibition of protein kinase C by calphostin C is light-dependent: R.F. Burns, et al.; BBRC 176, 288 (1991)
- Calphostin C, a specific protein kinase C inhibitor, activates human neutrophils: effect on phospholipase A2 and aggregation: S. Svetlov & S. Nigam; Biochim. Biophys. Acta 1177, 75 (1993)
- Inhibition of diacylglycerol kinase by the antitumor agent calphostin C. Evidence for similarity between the active site of diacylglycerol kinase and the regulatory site of protein kinase C: C. Redman, et al.; Biochem. Pharmacol. 50, 235 (1995)
- Calphostin C, a widely used protein kinase C inhibitor, directly and potently blocks L-type Ca channels: H.C. Hartzell & A. Rinderknecht; Am. J. Physiol. 270, C1293 (1996)
- Calphostin C synergistically induces apoptosis with VP-16 in lymphoma cells which express abundant phosphorylated Bcl-2 protein: M. Murata, et al.; Cell. Mol. Life Sci. 53, 737 (1997)
- Calphostin C induces selective disassembly of the Golgi complex by a protein kinase C-independent mechanism: M. Alonso, et al.; Eur. J. Cell Biol. 76, 93 (1998)
- Growth inhibition induced by Ro 31-8220 and calphostin C in human glioblastoma cell lines is associated with apoptosis and inhibition of CDC2 kinase: M Begemann, et al.; Anticancer Res. 18, 3139 (1998)
