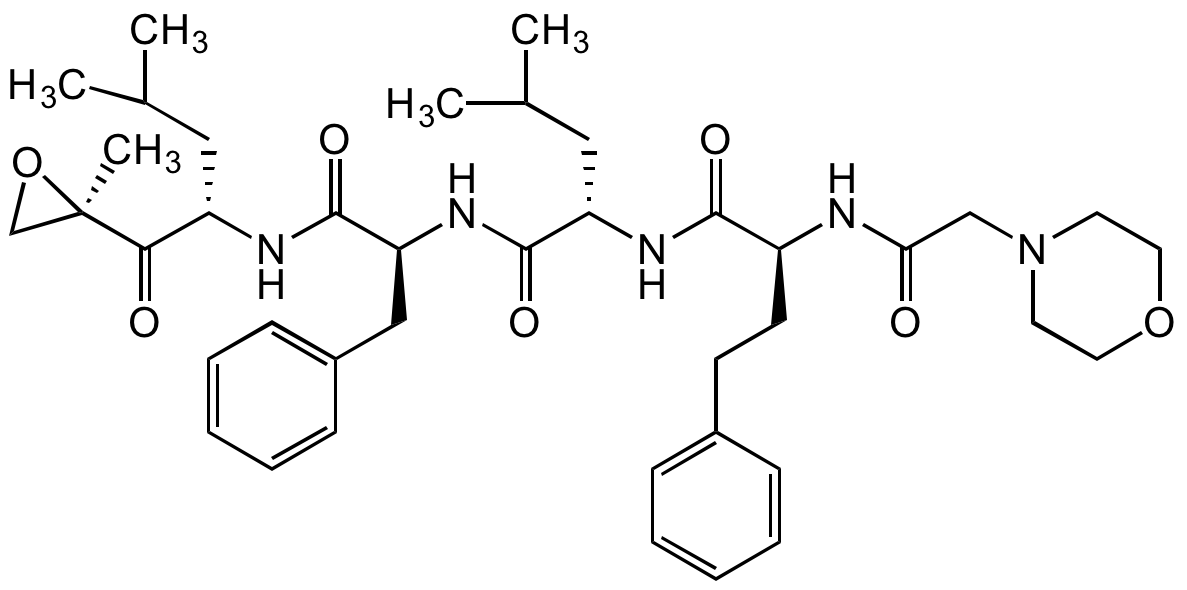
Chemical Structure
Carfilzomib [PR-171]

AG-CR1-3669
Overview
- SupplierAdipoGen Life Sciences
- Product NameCarfilzomib [PR-171] [868540-17-4]
- Delivery Days Customer10
- CAS Number868540-17-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger
- Molecular FormulaC40H57N5O7
- Molecular Weight719.9
- Scientific DescriptionChemical. CAS: 868540-17-4. Formula: C40H57N5O7. MW: 719.9. Synthetic. Potent and irreversible second-generation peptide epoxyketone class proteasome inhibitor. Synthetic analog of the microbial product epoxomcin. Targets the chymotrypsin-like beta5 subunit of the constitutive 20S proteasome (IC50=5.2nM) and the beta5i subunit [LMP7] of the 20S immunoproteasome (IC50=14nM), with minimal crossreactivity to other proteases. Displays equal potency but greater selectivity for the chymotrypsin-like activity of the proteasome compared to bortezomib. Anticancer compound effective against multiple myeloma in vivo. In vitro, induces cell cycle arrest and apoptosis in human cancer cell lines including multiple myeloma, lymphoma, and various solid tumors (IC50s=2.4-20nM). - Potent and irreversible second-generation peptide epoxyketone class proteasome inhibitor. Synthetic analog of the microbial product epoxomicin (Prod. No. AG-CN2-0422). Targets the chymotrypsin-like beta5 subunit of the constitutive 20S proteasome (IC50=5.2nM) and the beta5i subunit [LMP7] of the 20S immunoproteasome (IC50=14nM), with minimal cross-reactivity to other proteases. Displays equal potency but greater selectivity for the chymotrypsin-like activity of the proteasome compared to bortezomib (Prod. No. AG-CR1-3602). Anticancer compound effective against multiple myeloma in vivo. In vitro, induces cell cycle arrest and apoptosis in human cancer cell lines including multiple myeloma, lymphoma and various solid tumors (IC50s=2.4-20nM).
- SMILESO=C([C@]1(C)OC1)[C@H](CC(C)C)NC([C@H](CC2=CC=CC=C2)NC([C@H](CC(C)C)NC([C@H](CCC3=CC=CC=C3)NC(CN4CCOCC4)=O)=O)=O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome: S.D. Demo, et al.; Cancer Res. 67, 6383 (2007)
- Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma: D.J. Kuhn, et al.; Blood 110, 3281 (2007)
- The proteasome inhibitors bortezomib and PR-171 have antiproliferative and proapoptotic effects on primary human acute myeloid leukaemia cells: C. Stapnes, et al.; Br. J. Haematol. 136, 814 (2007)
- Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome: F. Parlati, et al.; Blood 114, 3439 (2009)
- Second generation proteasome inhibitors: carfilzomib and immunoproteasome-specific inhibitors (IPSIs): D.J. Kuhn, et al.; Curr. Cancer Drug Targets 11, 285 (2011) (Review)
- Carfilzomib-dependent selective inhibition of the chymotrypsin-like activity of the proteasome leads to antitumor activity in Waldenstrom's Macroglobulinemia: A. Sacco, et al.; Clin. Cancer Res. 17, 1753 (2011)
- Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events: S. Arastu-Kapur, et al.; Clin. Cancer Res. 17, 2734 (2011)
- Carfilzomib: a novel second-generation proteasome inhibitor: M.L. Khan & A.K. Stewart; Future Oncol. 7, 607 (2011)
- The next generation proteasome inhibitors carfilzomib and oprozomib activate prosurvival autophagy via induction of the unfolded protein response and ATF4: Y. Zang, et al.; Autophagy 8, 1873 (2012)
- From epoxomicin to carfilzomib: chemistry, biology, and medical outcomes: K.B. Kim & C.M. Crews; Nat. Prod. Rep. 30, 600 (2013)
