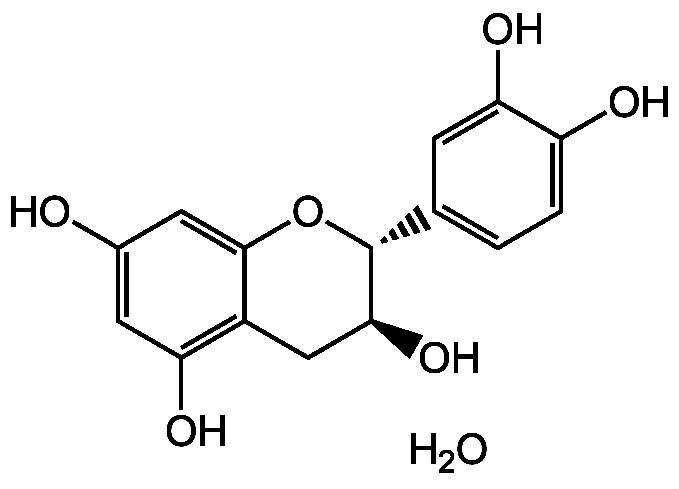
Chemical Structure
(+)-Catechin . hydrate [225937-10-0]

AG-CN2-0407
CAS Number225937-10-0
Product group Chemicals
Estimated Purity>95%
Molecular Weight290.3 . 18.0
Overview
- SupplierAdipoGen Life Sciences
- Product Name(+)-Catechin . hydrate [225937-10-0, 88191-48-4]
- Delivery Days Customer10
- CAS Number225937-10-0
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC15H14O6 . H2O
- Molecular Weight290.3 . 18.0
- Scientific DescriptionChemical. CAS: 225937-10-0 or 88191-48-4. Formula: C15H14O6 . H2O. MW: 290.3 . 18.0. Isolated from Uncaria rhynchophylla. Specific histidine decarboxylase inhibitor. Anti-inflammatory. COX-1 inhibitor. Antioxidant flavonoid. Free radical scavenger. Inhibits lipid peroxidation. Antimetastatic and anticancer compound. Antiangiogenic. Anti-osteoporotic. Antibacterial and antifungal. Neuroprotective. Mono oxidase B inhibitor. Apoptosis and cell cycle arrest inducer. JNK phosphorylation inhibitor. Antispasmodic. Insulin-mimetic. - Specific histidine decarboxylase inhibitor [1]. Anti-inflammatory. COX-1 inhibitor [2]. Antioxidant flavonoid. Free radical scavenger. Inhibits lipid peroxidation [3, 6, 9]. Antimetastatic and anticancer compound [4, 9, 10, 14, 16, 17]. Antiangiogenic [14]. Anti-osteoporotic [5]. Antibacterial and antifungal [7]. Neuroprotective [8]. Mono oxidase B inhibitor [11]. Apoptosis and cell cycle arrest inducer [9, 10, 16, 17]. JNK phosphorylation inhibitor [12]. Antispasmodic [13]. Insulin-mimetic [15].
- SMILESO.O[C@H]1CC2=C(O)C=C(O)C=C2O[C@@H]1C1=CC=C(O)C(O)=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Histamine and acute haemorrhagic lesions in rat gastric mucosa: prevention of stress ulcer formation by (+)-catechin, an inhibitor of specific histidine decarboxylase in vitro: H.J. Reimann, et al.; Agents Actions 7, 69 (1977)
- Flavan-3-ols isolated from some medicinal plants inhibiting COX-1 and COX-2 catalysed prostaglandin biosynthesis: Y. Noreen, et al.; Planta Med. 64, 520 (1998)
- (+)-Catechin as antioxidant: mechanisms preventing human plasma oxidation and activity in red wines: S.B. Lotito & C.G. Fraga; Biofactors 10, 125 (1999)
- Flavonoids as anticancer agents: structure-activity relationship study: M. Lopez-Lazaro; Curr. Med. Chem. Anticancer Agents 2, 691 (2002) (Review)
- Effects of (+)-catechin on the function of osteoblastic cells: E.M. Choi & J.K. Hwang; Biol. Pharm. Bull. 26, 523 (2003)
- Dietary supplementation of (+)-catechin protects against UVB-induced skin damage by modulating antioxidant enzyme activities: S.E. Jeon, et al.; Photodermatol. Photoimmunol. Photomed. 19, 235 (2003)
- Phytotoxic and antimicrobial activities of catechin derivatives: R. Veluri, et al.; J. Agric. Food Chem. 52, 1077 (2004)
- Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases: S. Mandel & M.B. Youdim; Free Radic. Biol. Med. 37, 304 (2004) (Review)
- A green tea component, catechin, rapidly induces apoptosis of myeloid leukemic cells via modulation of reactive oxygen species production in vitro and inhibits tumor growth in vivo: L. Gordon; Haematologica 90, 290 (2005)
- (+)-Catechin, an ingredient of green tea, protects murine microglia from oxidative stress-induced DNA damage and cell cycle arrest: Q. Huang, et al.; J. Pharmacol. Sci. 98, 16 (2005)
