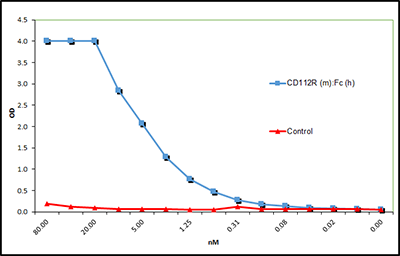
CD112R (mouse):Fc (human) (rec.)
AG-40B-0170
Product group Proteins / Signaling Molecules
Overview
- SupplierAdipoGen Life Sciences
- Product NameCD112R (mouse):Fc (human) (rec.)
- Delivery Days Customer10
- CertificationResearch Use Only
- Concentration0.1 mg/ml
- Estimated Purity>90%
- Scientific DescriptionCD112 (also called Nectin-2 or poliovirus receptor-related 2 protein) is a type I transmembrane glycoprotein and a member of the Ig gene superfamily (IGSF). CD112/Nectin-2 functions as an adhesion molecule involved in the formation of cell junctions and interactions with other Nectin-family molecules. Nectin-2 is a also a surface marker of some endothelial cells and regulates migration, proliferation and angiogenesis. CD112 functions as an immune checkpoint regulator by modulating T cell signaling, either as a costimulator of T cell function, or as a coinhibitor of T cells proliferation by binding to the receptors CD226 or TIGIT (T cell Ig and immunoreceptor tyrosine-based inhibitory motif (ITIM) domain), respectively. The binding of CD112 ligand to these receptors is weak compared to CD155. Recently, a new receptor of CD112, called CD112R or PVRIG (Poliovirus receptor-related immunoglobulin domain-containing protein) has been characterized, showing high affinity to CD112. CD112R is a new coinhibitory receptor of the PVR family for human T cells, inhibiting TCR-mediated signals upon binding to CD112/Nectin-2. CD112R is preferentially expressed in T lymphocytes and NK cells. Cancer cells evade immune responses as a result of an immunosuppressive tumor microenvironment and CD112R, by playing a role in the regulation of the immune system, could be a new attractive cancer immunotherapy target. - Protein. The extracellular domain of mouse CD112R (aa 28-165) is fused at the C-terminus to the Fc portion of human IgG1. Source: HEK 293 cells. Lyophilized. Contains PBS. Purity: >90% (SDS-PAGE). CD112 (also called Nectin-2 or poliovirus receptor-related 2 protein) is a type I transmembrane glycoprotein and a member of the Ig gene superfamily (IGSF). CD112/Nectin-2 functions as an adhesion molecule involved in the formation of cell junctions and interactions with other Nectin-family molecules. Nectin-2 is a also a surface marker of some endothelial cells and regulates migration, proliferation and angiogenesis. CD112 functions as an immune checkpoint regulator by modulating T cell signaling, either as a costimulator of T cell function, or as a coinhibitor of T cells proliferation by binding to the receptors CD226 or TIGIT (T cell Ig and immunoreceptor tyrosine-based inhibitory motif (ITIM) domain), respectively. The binding of CD112 ligand to these receptors is weak compared to CD155. Recently, a new receptor of CD112, called CD112R or PVRIG (Poliovirus receptor-related immunoglobulin domain-containing protein) has been characterized, showing high affinity to CD112. CD112R is a new coinhibitory receptor of the PVR family for human T cells, inhibiting TCR-mediated signals upon binding to CD112/Nectin-2. CD112R is preferentially expressed in T lymphocytes and NK cells. Cancer cells evade immune responses as a result of an immunosuppressive tumor microenvironment and CD112R, by playing a role in the regulation of the immune system, could be a new attractive cancer immunotherapy target.
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116100
