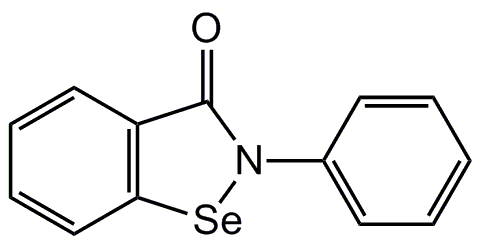
Chemical Structure
Ebselen [60940-34-3]

AG-CR1-0031
CAS Number60940-34-3
Product group Chemicals
Estimated Purity>98%
Molecular Weight274.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameEbselen [60940-34-3]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number60940-34-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC13H9NOSe
- Molecular Weight274.2
- Scientific DescriptionChemical. CAS: 60940-34-3. Formula: C13H9NOSe. MW: 274.2. Glutathione peroxidase mimetic. Peroxynitrite scavenger. Anti-inflammatory Antioxidant. Protein kinase C (PKC), NADPH, lipoxygenase, COX, NOS, H+-ATPase and NADPH oxidase inhibitor. Antifungal. Review. Potent IDO-1 inhibitor with Ki 94nM. Inhibitor of HIV-1 capsid C-terminal domain dimerization. - Glutathione peroxidase mimetic [1, 4]. Peroxynitrite scavenger [4]. Anti-inflammatory [1, 5-7] Antioxidant [2, 3, 5]. Protein kinase C (PKC), NADPH, lipoxygenase, COX, NOS, H+-ATPase and NADPH oxidase inhibitor [6-9]. Antifungal [9]. Review [10]. Potent IDO-1 inhibitor with Ki 94nM [11]. Inhibitor of HIV-1 capsid C-terminal domain dimerization. Shown to potentially inhibit the 33.8-kDa Main Protease (Mpro)/3C-like Protease of SARS-CoV-2, consequently inhibiting viral transcription and replication and possibly inhibiting spread of COVID-19.
- SMILESO=C1N([Se]C2=C1C=CC=C2)C1=CC=CC=C1
- Storage Instruction2°C to 8°C,-20°C
- UN NumberUN 3283
- UNSPSC12352200
References
- A novel biologically active seleno-organic compound--III. Effects of PZ 51 (Ebselen) on glutathione peroxidase and secretory activities of mouse macrophages: M.J. Parnham & S. Kindt; Biochem. Pharmacol. 33, 3247 (1984)
- Seleno-organic compounds and the therapy of hydroperoxide-linked pathological conditions: M.J. Parnham & E. Graf; Biochem. Pharmacol. 36, 3095 (1987)
- Kinetic mechanism and substrate specificity of glutathione peroxidase activity of ebselen (PZ51): M. Maiorino, et al.; Biochem. Pharmacol. 37, 2267 (1988)
- Ebselen as a glutathione peroxidase mimic and as a scavenger of peroxynitrite: H. Sies & H. Masumoto; Adv. Pharmacol.38, 229 (1997)
- Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis: Y. Nakamura, et al.; J. Biol. Chem. 277, 2687 (2002)
- Molecular actions of ebselen-an antiinflammatory antioxidant: T. Schewe; Gen. Pharmacol. 26, 1153 (1995)
- Studies on the anti-inflammatory activity of ebselen. Ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C?: I.A. Cotgreave, et al.; Biochem. Pharmacol. 38, 649 (1989)
- Strong inhibition of mammalian lipoxygenases by the antiinflammatory seleno-organic compound ebselen in the absence of glutathione: C. Schewe, et al.; Biochem. Pharmacol. 48, 65 (1994)
- Evaluation of the antifungal and plasma membrane H+-ATPase inhibitory action of ebselen and two ebselen analogs in S. cerevisiae cultures: B. Billack, et al.; J. Enzyme Inhib. Med. Chem. 25, 312 (2010)
- Ebselen: a thioredoxin reductase-dependent catalyst for alpha-tocopherol quinone reduction: J. Fang, et al.; Toxicol. Appl. Pharmacol. 207, 103 (2005)
