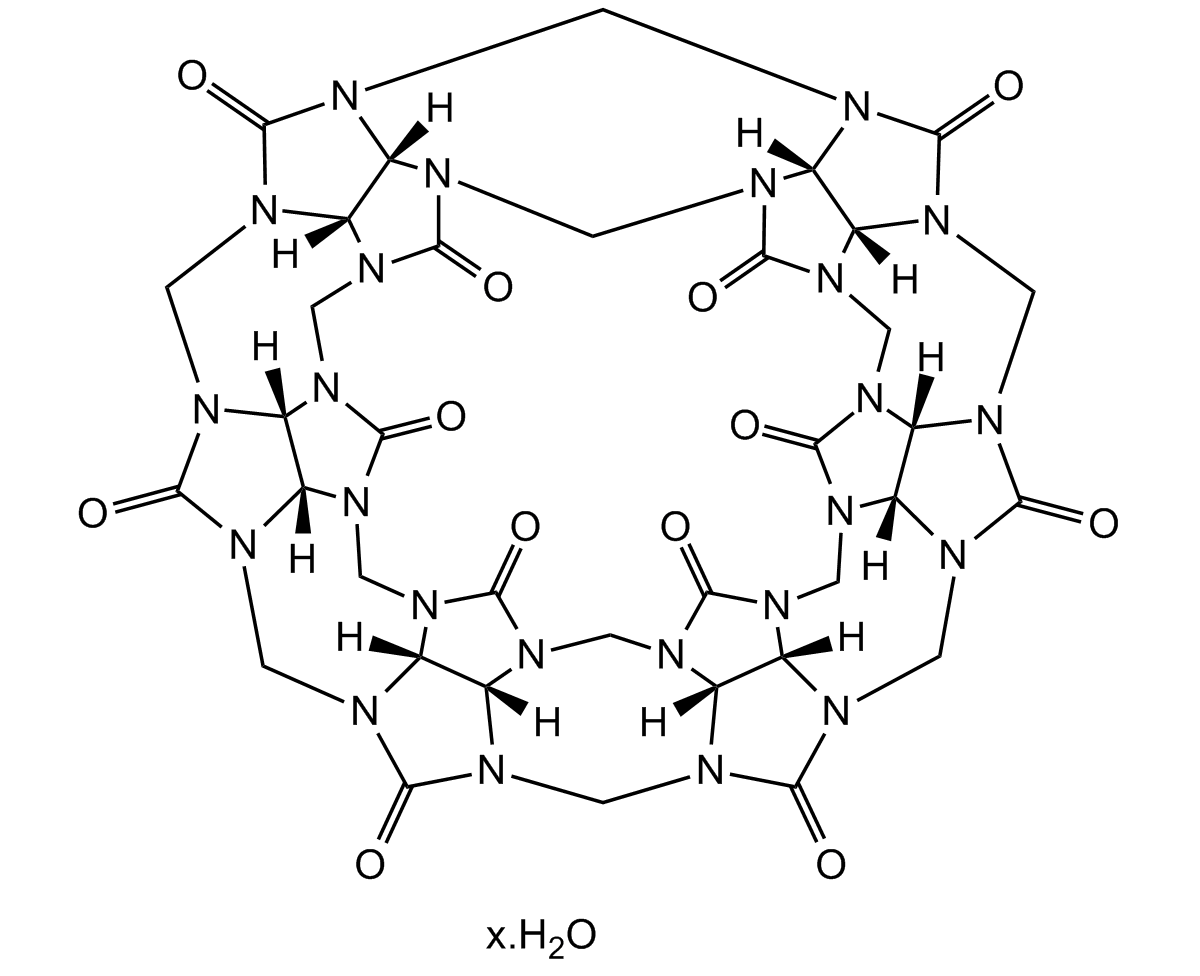
Chemical Structure
Cucurbit[6]uril hydrate [80262-44-8] [80262-44-8]
CDX-C0239
CAS Number80262-44-8
Product group Chemicals
Estimated Purity>95% (N)
Molecular Weight996.84
Overview
- SupplierChemodex
- Product NameCucurbit[6]uril hydrate [80262-44-8] [80262-44-8]
- Delivery Days Customer10
- CAS Number80262-44-8
- CertificationResearch Use Only
- Estimated Purity>95% (N)
- Molecular FormulaC36H36N24O12
- Molecular Weight996.84
- Scientific DescriptionChemical. CAS: 80262-44-8. Formula: C36H36N24O12. MW: 996.84. Cucurbit[6]uril hydrate belongs to the Cucurbit[n]uril (CB[n], n = 5-10) family of macrocyclic compounds comprising n glycoluril units, self-assembled from an acid-catalyzed condensation reaction of glycoluril and formaldehyde. The pumpkin-shaped CB molecules have a hydrophobic cavity and two identical carbonyllaced portals. While the hydrophobic interior provides a potential inclusion site for nonpolar molecules, the polar ureido carbonyl groups at the portals allow CB[n] to bind ions and molecules through charge-dipole and hydrogen bonding interactions. The unique structure and recognition properties make CB[n] attractive not only as a synthetic receptor but also as a building block for the construction of supramolecular architectures. In the wide area of supramolecular chemistry, cucurbit[n]urils (CBn) present themselves as molecular containers, able to form stable complexes with various guests, including drug molecules, amino acids and peptides, saccharides, dyes, hydrocarbons, perfluorinated hydrocarbons, and even high molecular weight guests such as proteins (e.g. human insulin). Furthermore, a direct functionalization method of CB[n] allowed the synthesis of a wide variety of tailor-made CB derivatives to study many applications. Ion channels, vesicles, polymers, nanomaterials, ion selective electrodes incorporating CB[n], and CB-immobilized solid surfaces and silica gel have been reported. Numerous other applications are being explored. - Cucurbit[6]uril hydrate belongs to the Cucurbit[n]uril (CB[n], n = 5-10) family of macrocyclic compounds comprising n glycoluril units, self-assembled from an acid-catalyzed condensation reaction of glycoluril and formaldehyde. The pumpkin-shaped CB molecules have a hydrophobic cavity and two identical carbonyllaced portals. While the hydrophobic interior provides a potential inclusion site for nonpolar molecules, the polar ureido carbonyl groups at the portals allow CB[n] to bind ions and molecules through charge-dipole and hydrogen bonding interactions. The unique structure and recognition properties make CB[n] attractive not only as a synthetic receptor but also as a building block for the construction of supramolecular architectures. In the wide area of supramolecular chemistry, cucurbit[n]urils (CBn) present themselves as molecular containers, able to form stable complexes with various guests, including drug molecules, amino acids and peptides, saccharides, dyes, hydrocarbons, perfluorinated hydrocarbons, and even high molecular weight guests such as proteins (e.g. human insulin). Furthermore, a direct functionalization method of CB[n] allowed the synthesis of a wide variety of tailor-made CB derivatives to study many applications. Ion channels, vesicles, polymers, nanomaterials, ion selective electrodes incorporating CB[n], and CB-immobilized solid surfaces and silica gel have been reported. Numerous other applications are being explored.
- SMILESO=C(N(C1)[C@@]2([H])N3CN4[C@@]5([H])N1C(N(CN6[C@]7([H])N8C(N(C9)[C@]7([H])N(C%10)C6=O)=O)[C@@]5([H])N(C8)C4=O)=O)N(CN%11[C@@]%12([H])N%13C(N(C%14)[C@@]%12([H])N(CN%15[C@@]%16([H])N%14C(N(C%17)[C@@]%16([H])N(CN%18[C@@]%19([H])N%17C(N9[C@@]%19([H])N%10C%18=O)=O)C%15=O)=O)C%11=O)=O)[C@]2([H])N(C%13)C3=O
- Storage InstructionRT
- UNSPSC12352200
