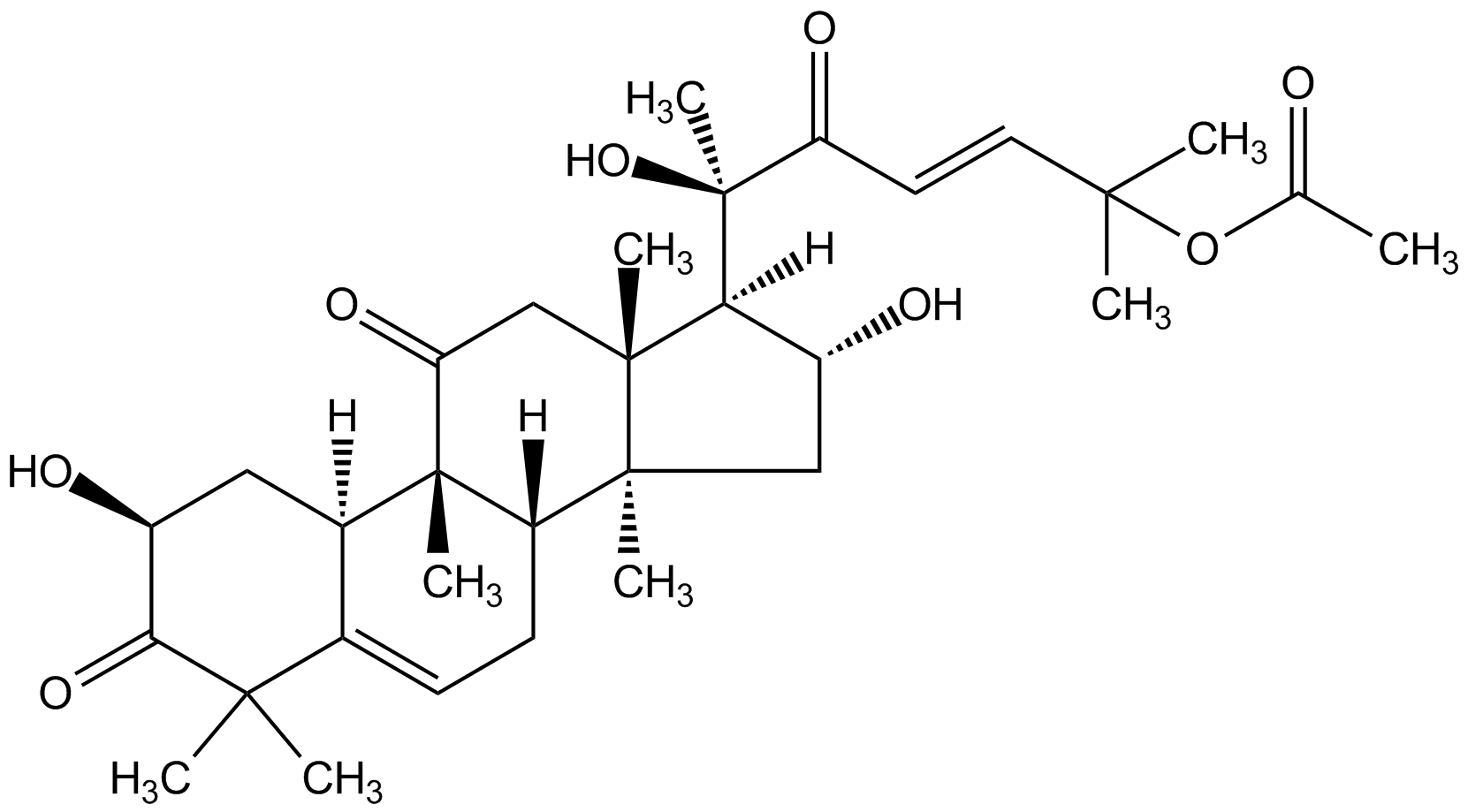
Chemical Structure
Cucurbitacin B [6199-67-3]
AG-CN2-0472
CAS Number6199-67-3
Product group Chemicals
Estimated Purity>98%
Molecular Weight558.7
Overview
- SupplierAdipoGen Life Sciences
- Product NameCucurbitacin B [6199-67-3]
- Delivery Days Customer10
- CAS Number6199-67-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC32H46O8
- Molecular Weight558.7
- Scientific DescriptionChemical. CAS: 6199-67-3. Formula: C32H46O8. MW: 558.7. Isolated from Cucumis melo L. Microtubule polymerization inhibitor. Disrupts F-actin and induces nucleophosmin/B23 translocation. Immunomodulator with antimicrobial, anti-inflammatory and antitumorigenic properties. Induces apoptosis, autophagy and cell cycle arrest at G2/M phase in a range of cancer cell lines. Shown to inhibit STAT 3 phosphorylation and expression levels and blocks JAK2 activity, as well as the transcriptional activity of HIF1alpha and NF-kappaB. Antioxidant. Serves as an ecdysteroid receptor antagonist in Drosophila. - Microtubule polymerization inhibitor. Disrupts F-actin and induces nucleophosmin/B23 translocation. Immunomodulator with antimicrobial, anti-inflammatory and antitumorigenic properties. Induces apoptosis, autophagy and cell cycle arrest at G2/M phase in a range of cancer cell lines. Shown to inhibit STAT 3 phosphorylation and expression levels and blocks JAK2 activity, as well as the transcriptional activity of HIF1alpha and NF-kappaB. Antioxidant. Serves as an ecdysteroid receptor antagonist in Drosophila.
- SMILES[H][C@@]1([C@H](O)C[C@@]2(C)[C@]3([H])CC=C4[C@@]([H])(C[C@H](O)C(=O)C4(C)C)[C@]3(C)C(=O)C[C@]12C)[C@@](C)(O)C(=O)\C=C\C(C)(C)OC(C)=O
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200


![Cucurbitacin B [6199-67-3]](https://www.targetmol.com/group3/M00/35/77/CgoaEGayIAWECqXBAAAAAEvHz5c340.png)