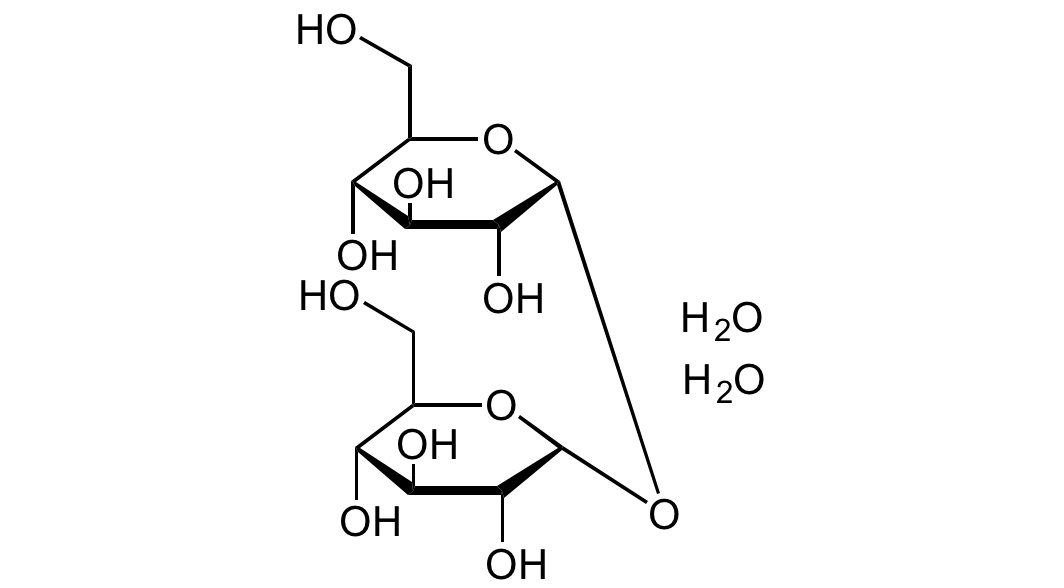
Chemical Structure
D-(+)-Trehalose dihydrate [6138-23-4] [6138-23-4]
CDX-T0202
CAS Number6138-23-4
Product group Chemicals
Estimated Purity>99%
Molecular Weight378.33
Overview
- SupplierChemodex
- Product NameD-(+)-Trehalose dihydrate [6138-23-4] [6138-23-4]
- Delivery Days Customer2
- CAS Number6138-23-4
- CertificationResearch Use Only
- Estimated Purity>99%
- Molecular FormulaC12H22O11 . 2H2O
- Molecular Weight378.33
- Scientific DescriptionChemical. CAS: 6138-23-4. Formula: C12H22O11 . 2H2O. MW: 378.33. Synthetic. Natural alpha-linked disaccharide formed by alpha-glucose units. Can be synthesized by bacteria, fungi, plants and invertebrate animals. It is implicated in anhydrobiosis, the ability of plants and animals to withstand prolonged periods of desiccation/dehydration. The sugar is thought to form a gel phase as cells dehydrate, which prevents disruption of internal cell organelles. Rehydration then allows normal cellular activity to be resumed without the major, lethal damage that would normally follow a dehydration/rehydration cycle. It has high water retention capabilities, and is used in food and cosmetics. Used as a cryoprotectant in a variety of cell freezing media, in solid dosage formulations, most notably in quick-dissolving tablets and to preserve foods, enzymes, vaccines, cells in a dehydrated state at room temperature. Major carbohydrate energy storage molecule used by insects for flight. Osmolyte, a chemical chaperone and inducer of autophagy, that has been reported to protect cells against numerous environmental stresses. This compound contains many properties that allow it to stabilize partially unfolded protein molecules and inhibit protein aggregation. - Natural alpha-linked disaccharide formed by alpha-glucose units. Can be synthesized by bacteria, fungi, plants and invertebrate animals. It is implicated in anhydrobiosis, the ability of plants and animals to withstand prolonged periods of desiccation/dehydration. The sugar is thought to form a gel phase as cells dehydrate, which prevents disruption of internal cell organelles. Rehydration then allows normal cellular activity to be resumed without the major, lethal damage that would normally follow a dehydration/rehydration cycle. It has high water retention capabilities, and is used in food and cosmetics. Used as a cryoprotectant in a variety of cell freezing media, in solid dosage formulations, most notably in quick-dissolving tablets and to preserve foods, enzymes, vaccines, cells in a dehydrated state at room temperature. Major carbohydrate energy storage molecule used by insects for flight. Osmolyte, a chemical chaperone and inducer of autophagy, that has been reported to protect cells against numerous environmental stresses. This compound contains many properties that allow it to stabilize partially unfolded protein molecules and inhibit protein aggregation.
- SMILESOC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@H]2[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O2)O1.O.O
- Storage Instruction2°C to 8°C,RT
- UNSPSC12352200



![D-(+)-Trehalose dihydrate [6138-23-4] [6138-23-4]](https://www.targetmol.com/group3/M00/03/47/CgoaEGY7TNqERzAIAAAAAB0R5Qs451.png)