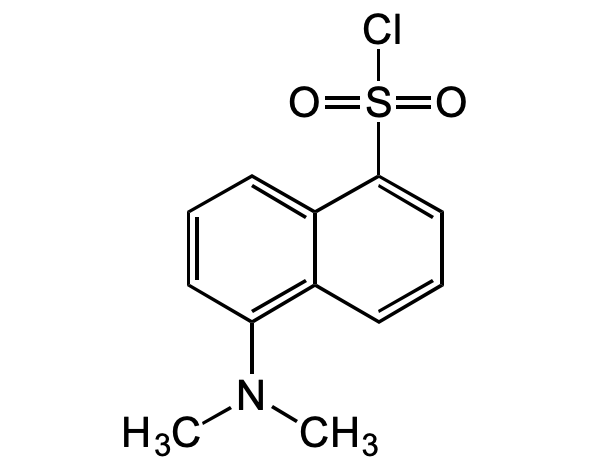
Chemical Structure
Dansyl chloride [605-65-2]
CDX-D0043
CAS Number605-65-2
Product group Chemicals
Estimated Purity>98%
Molecular Weight269.75
Overview
- SupplierChemodex
- Product NameDansyl chloride [605-65-2]
- Delivery Days Customer10
- CAS Number605-65-2
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC12H12ClNO2S
- Molecular Weight269.75
- Scientific DescriptionChemical. CAS: 605-65-2. Formula: C12H12ClNO2S. MW: 269.75. Synthetic. Dansyl chloride is a fluorogenic probe that reacts with primary amino groups in both aliphatic and aromatic amines to produce stable blue- or blue-green-fluorescent sulfonamide adducts. It is used for protein sequencing and N-terminal amino acid and peptide analysis by reverse phase high performance liquid chromatography (HPLC). It can also be used to label hydroxyl and carboxylic acid functional groups. Dansyl chloride is non-fluorescent until it reacts with amines. The resulting Dansyl amides have environmentally sensitive fluorescence quantum yields and emission maxima along with large Stokes shifts. This environment-sensitive fluorescence property, in combination with their ability to accept energy (as in fluorescence resonance energy transfer) from the amino acid tryptophan, has made Dansyl chloride an important tool for biophysical studies. It is particularly useful for preparing fluorescent drug or ligand analogs that are expected to bind to hydrophobic sites in proteins or membranes or other biological receptors. Dansyl protein conjugates have fluorescence lifetimes of 10-20 nanoseconds. Spectral Date in DMF: Excitation/Emission ~333nm/515nm. Dansyl chloride is unstable in dimethyl sulfoxide, which should never be used to prepare solutions of the reagent. Dansyl chloride is one of the simplest sulfonamide derivatives, so it commonly serves as a starting reagent for the production of other derivatives. - Dansyl chloride is a fluorogenic probe that reacts with primary amino groups in both aliphatic and aromatic amines to produce stable blue- or blue-green-fluorescent sulfonamide adducts. It is used for protein sequencing and N-terminal amino acid and peptide analysis by reverse phase high performance liquid chromatography (HPLC). It can also be used to label hydroxyl and carboxylic acid functional groups. Dansyl chloride is non-fluorescent until it reacts with amines. The resulting Dansyl amides have environmentally sensitive fluorescence quantum yields and emission maxima along with large Stokes shifts. This environment-sensitive fluorescence property, in combination with their ability to accept energy (as in fluorescence resonance energy transfer) from the amino acid tryptophan, has made Dansyl chloride an important tool for biophysical studies. It is particularly useful for preparing fluorescent drug or ligand analogs that are expected to bind to hydrophobic sites in proteins or membranes or other biological receptors. Dansyl protein conjugates have fluorescence lifetimes of 10-20 nanoseconds. Spectral Date in DMF: Excitation/Emission ~333nm/515nm. Dansyl chloride is unstable in dimethyl sulfoxide, which should never be used to prepare solutions of the reagent. Dansyl chloride is one of the simplest sulfonamide derivatives, so it commonly serves as a starting reagent for the production of other derivatives.
- SMILESCN(C)C1=C(C=CC=C2S(=O)(Cl)=O)C2=CC=C1
- Storage Instruction2°C to 8°C
- UN Number3261
- UNSPSC12162000

![Dansyl chloride [605-65-2]](https://www.targetmol.com/group3/M00/35/C7/CgoaEGayK2SEEi5PAAAAAM_OAR8959.png)