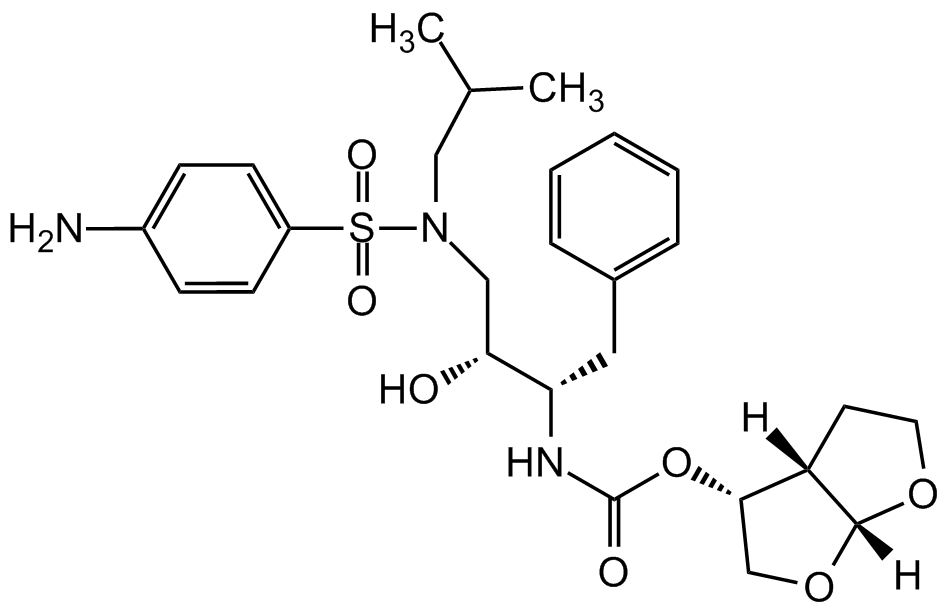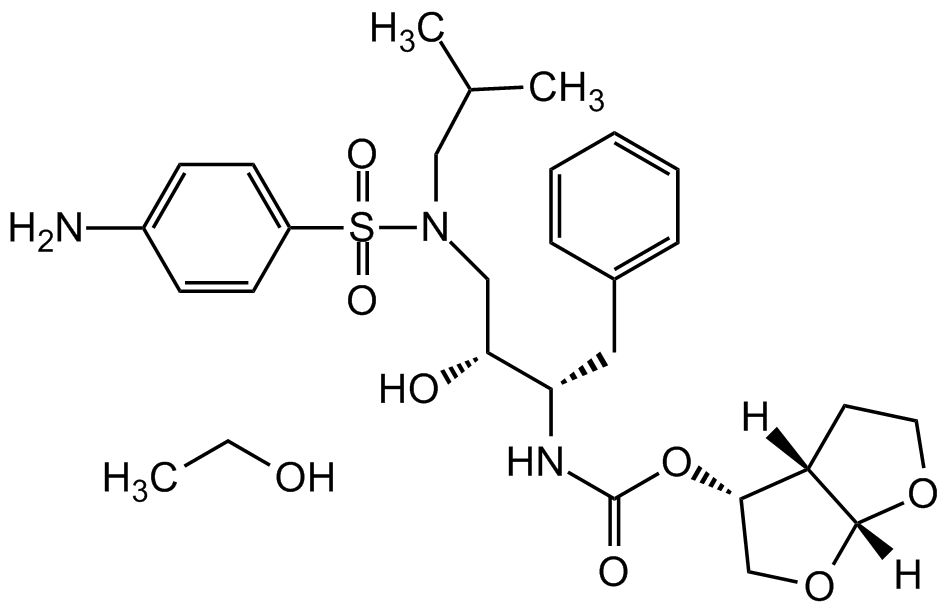
Chemical Structure
Darunavir . ethanolate
AG-CR1-3724
CAS Number635728-49-3
Product group Chemicals
Estimated Purity>98%
Molecular Weight547.7 . 46.0
Overview
- SupplierAdipoGen Life Sciences
- Product NameDarunavir . ethanolate
- Delivery Days Customer10
- CAS Number635728-49-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC27H37N3O7S . C2H5OH
- Molecular Weight547.7 . 46.0
- Scientific DescriptionChemical. CAS: 635728-49-3. Formula: C27H37N3O7S . C2H5OH. MW: 547.7 . 46.0. Darunavir ethanolate is a highly potent HIV protease inhibitor (IC50=3-6nM, depending on laboratory HIV-1 strain), an antiretroviral medication used to treat and prevent HIV/AIDS. The ethanolate formulation has some stability advantages compared to the free base Darunavir (Prod. No. AG-CR1-3712). Darunavir ethanolate is a second generation HIV-1 protease inhibitor that inhibits replication of various laboratory strains and clinical isolates of HIV-1, including those resistant to first generation protease inhibitors. It inhibits cell-free diffusion and cell-to-cell spread of HIV-1 in Jurkat cell populations. Formulations containing darunavir have been used in combination therapy for the treatment of HIV. Shown in a SARS-CoV-2 protease structure model study to potentially bind and inhibit the papain like viral protease (PLVP) of SARS-CoV-2, responsible for COVID-19. - Darunavir ethanolate is a highly potent HIV protease inhibitor (IC50=3-6nM, depending on laboratory HIV-1 strain), an antiretroviral medication used to treat and prevent HIV/AIDS. The ethanolate formulation has some stability advantages compared to the free base Darunavir (Prod. No. AG-CR1-3712). Darunavir ethanolate is a second generation HIV-1 protease inhibitor that inhibits replication of various laboratory strains and clinical isolates of HIV-1, including those resistant to first generation protease inhibitors. It inhibits cell-free diffusion and cell-to-cell spread of HIV-1 in Jurkat cell populations. Formulations containing darunavir have been used in combination therapy for the treatment of HIV. Shown in a SARS-CoV-2 protease structure model study to potentially bind and inhibit the papain like viral protease (PLVP) of SARS-CoV-2, responsible for COVID-19.
- SMILESNC1=CC=C(S(=O)(N(C[C@@H](O)[C@H](CC2=CC=CC=C2)NC(O[C@H]3CO[C@]4([H])[C@@]3([H])CCO4)=O)CC(C)C)=O)C=C1.CCO
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Darunavir Ethanolate [635728-49-3]](https://www.targetmol.com/group3/M00/35/45/CgoaEWayGsOEMmLSAAAAADAvHC4556.png)