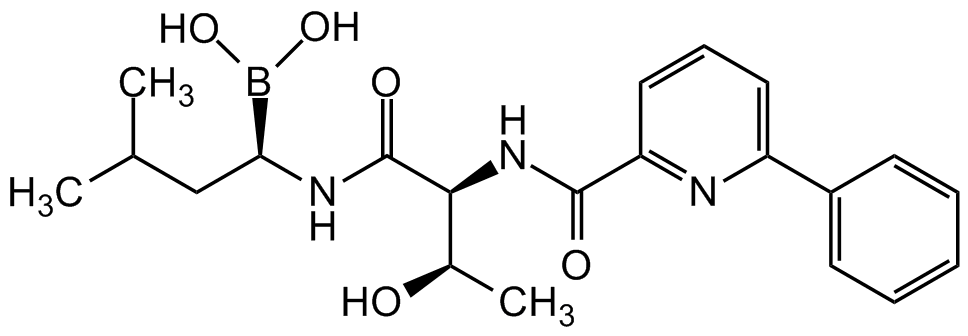
Chemical Structure
Delanzomib [CEP-18770]

AG-CR1-3673
Overview
- SupplierAdipoGen Life Sciences
- Product NameDelanzomib [CEP-18770] [847499-27-8]
- Delivery Days Customer10
- CAS Number847499-27-8
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC21H28BN3O5
- Molecular Weight413.3
- Scientific DescriptionChemical. CAS: 847499-27-8. Formula: C21H28BN3O5. MW: 413.3. Synthetic. Potent, selective, reversible orally bioavailable proteasome inhibitor. Synthetic P2 threonine boronic acid. Targets the chymotrypsin-like beta5 subunit of the constitutive 20S proteasome (IC50=3.8nM). Cross-reacts and inhibits the caspase-like/peptidyl-glutamyl peptide-hydrolyzing (PGPH) beta1 subunit (IC50=~70nM). Displays similar potency for the chymotrypsin-like activity of the proteasome compared to bortezomib. Exhibits a favorable cytotoxicity profile toward normal human epithelial cells, bone marrow progenitors and bone marrow-derived stromal cells relative to bortezomib. Anticancer compound effective against multiple myeloma in vivo. In vitro, blocks the growth of representative human solid and hematological tumor cell lines (IC50s=5.6-34nM). Shown to down-modulate NF-kappaB, induce apoptosis, inhibit angiogenesis and M-CSF-RANKL-induced osteoclastogenesis. - Potent, selective, reversible orally bioavailable proteasome inhibitor. Synthetic P2 threonine boronic acid. Targets the chymotrypsin-like beta5 subunit of the constitutive 20S proteasome (IC50=3.8nM). Cross-reacts and inhibits the caspase-like/peptidyl-glutamyl peptide-hydrolyzing (PGPH) beta1 subunit (IC50=~70nM). Displays similar potency for the chymotrypsin-like activity of the proteasome compared to bortezomib. Exhibits a favorable cytotoxicity profile toward normal human epithelial cells, bone marrow progenitors and bone marrow-derived stromal cells relative to bortezomib (Prod. No. AG-CR1-3602). Anticancer compound effective against multiple myeloma in vivo. In vitro, blocks the growth of representative human solid and hematological tumor cell lines (IC50s=5.6-34nM). Shown to down-modulate NF-kappaB, induce apoptosis, inhibit angiogenesis and M-CSF-RANKL-induced osteoclastogenesis.
- SMILESCC(C)C[C@@H](B(O)O)NC([C@H]([C@H](O)C)NC(C1=NC(C2=CC=CC=C2)=CC=C1)=O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Discovery of a Potent, Selective, and Orally Active Proteasome Inhibitor for the Treatment of Cancer: B.D. Dorsey, et al.; J. Med. Chem. 51, 1068 (2008)
- CEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib: R. Piva, et al.; Blood 111, 2765 (2008)
- The proteasome inhibitor CEP-18770 enhances the anti-myeloma activity of bortezomib and melphalan: E. Sanchez, et al.; Br. J. Haematol. 148, 569 (2010)
- Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE: M.M. Seavey, et al.; Int. Immunopharmacol. 12, 257 (2012)
- Probing the specificity and activity profiles of the proteasome inhibitors bortezomib and delanzomib: C.R. Berkers, et al.; Mol. Pharm. 9, 1126 (2012)
- Molecular mechanisms of acquired proteasome inhibitor resistance: A.J. Kale & B.S. Moore; J. Med. Chem. 55, 10317 (2012)
- A first in human phase I study of the proteasome inhibitor CEP-18770 in patients with advanced solid tumours and multiple myeloma: E. Gallerani, et al.; Eur. J. Cancer 49, 290 (2013)
- Proteasome inhibitors in the treatment of multiple myeloma: A. McBride & P.Y. Ryan; Expert Rev. Anticancer Ther. 13, 339 (2013) (Review)
- Phase I/II study of the novel proteasome inhibitor delanzomib (CEP-18770) for relapsed and refractory multiple myeloma: D.T. Vogl, et al.; Leuk. Lymphoma 58, 1872 (2017)
