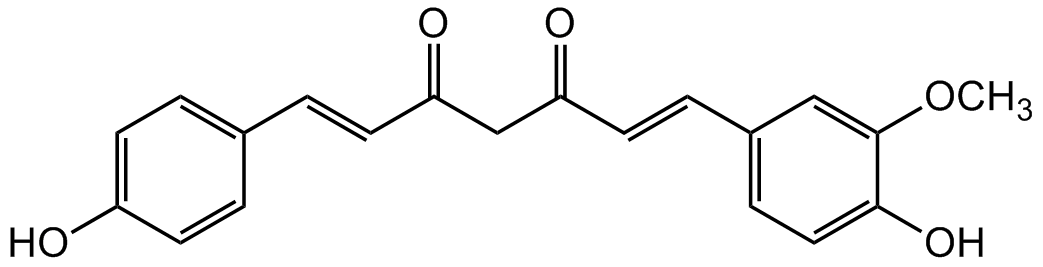
Chemical Structure
Demethoxycurcumin [22608-11-3] [22608-11-3]
CDX-D0929
CAS Number22608-11-3
Product group Chemicals
Estimated Purity>98%
Molecular Weight338.35
Overview
- SupplierChemodex
- Product NameDemethoxycurcumin [22608-11-3] [22608-11-3]
- Delivery Days Customer2
- CAS Number22608-11-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC20H18O5
- Molecular Weight338.35
- Scientific DescriptionChemical. CAS: 22608-11-3. Formula: C20H18O5. MW: 338.35. Demethoxycurcumin (DMC) is a natural demethoxy derivative of curcumin with anti-inflammatory and anti-cancer properties. DMC suppresses cell proliferation, migration and invasion in cancer cells. It down-regulates the transcriptional coactivator p300, suppressing the Wnt/beta-catenin pathway, and inhibits lipopolysaccharide induction of iNOS by blocking NF-kappa activation. It also has been shown to inhibit energy metabolic and oncogenic signaling pathways through AMPK activation. In addition it is a potent inhibitor of P-Type ATPases. Shown to have neuroprotective properties. It also has anti-plasmodial activity. - Demethoxycurcumin (DMC) is a natural demethoxy derivative of curcumin with anti-inflammatory and anti-cancer properties. DMC suppresses cell proliferation, migration and invasion in cancer cells. It down-regulates the transcriptional coactivator p300, suppressing the Wnt/beta-catenin pathway, and inhibits lipopolysaccharide induction of iNOS by blocking NF-kappa activation. It also has been shown to inhibit energy metabolic and oncogenic signaling pathways through AMPK activation. In addition it is a potent inhibitor of P-Type ATPases. Shown to have neuroprotective properties. It also has anti-plasmodial activity.
- SMILESOC1=CC=C(/C=C/C(CC(/C=C/C2=CC=C(O)C(OC)=C2)=O)=O)C=C1
- Storage Instruction2°C to 8°C,RT
- UN Number3077
- UNSPSC12352200


![Demethoxycurcumin [22608-11-3]](https://www.targetmol.com/group3/M00/35/AE/CgoaEWayKNaEQCsYAAAAAH_dkWg793.png)