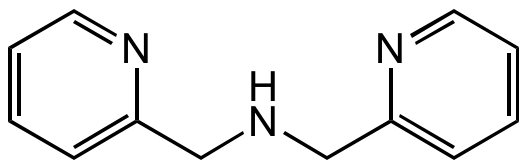
Chemical Structure
Di-(2-picolyl)amine [1539-42-0] [1539-42-0]
CDX-B0134
CAS Number1539-42-0
Product group Chemicals
Estimated Purity>95%
Molecular Weight199.25
Overview
- SupplierChemodex
- Product NameDi-(2-picolyl)amine [1539-42-0] [1539-42-0]
- Delivery Days Customer2
- CAS Number1539-42-0
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC12H13N3
- Molecular Weight199.25
- Scientific DescriptionBuilding block for synthesis. DPA is a secondary amine with two picolyl substituents. The compound is a tridentate ligand in coordination chemistry and commonly used to produce Zn-based chemosensors/probes, such as Zinpry. As a tridentate ligand this compound provides three nitrogen donors that affords good selectivity for Zn2+ over biologically relevant metals such as Na+, K+, Mg2+ and Ca2+, and leaves coordination sites free for anion binding. The amino nitrogen of the DPA group is a good candidate as an electron donor in either photoinduced electron transfer or photoinduced charge transfer (PET or PCT) sensors. Zn(II)-DPA complexes are widely used in anion recognition and sensing. - Chemical. CAS: 1539-42-0. Formula: C12H13N3. MW: 199.25. Synthetic Building block for synthesis. DPA is a secondary amine with two picolyl substituents. The compound is a tridentate ligand in coordination chemistry and commonly used to produce Zn-based chemosensors/probes, such as Zinpry. As a tridentate ligand this compound provides three nitrogen donors that affords good selectivity for Zn2+ over biologically relevant metals such as Na+, K+, Mg2+ and Ca2+, and leaves coordination sites free for anion binding. The amino nitrogen of the DPA group is a good candidate as an electron donor in either photoinduced electron transfer or photoinduced charge transfer (PET or PCT) sensors. Zn(II)-DPA complexes are widely used in anion recognition and sensing.
- SMILESC(NCC1=CC=CC=N1)C1=CC=CC=N1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200