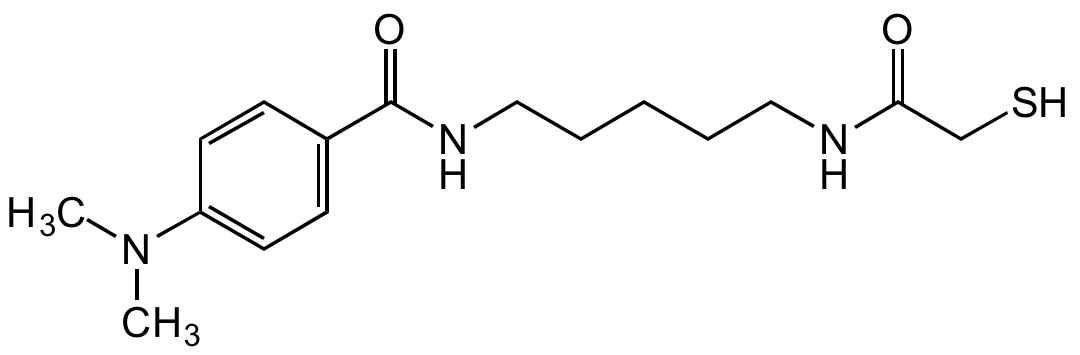
Chemical Structure
DMAPB [827036-76-0]

AG-CR1-3904
Overview
- SupplierAdipoGen Life Sciences
- Product NameDMAPB [827036-76-0]
- Delivery Days Customer10
- CAS Number827036-76-0
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC16H25N3O2S
- Molecular Weight323.5
- Scientific DescriptionCell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =114nM). Displays selectivity over other HDACs (IC50=1-8microM). Neuroprotective and anti-neuroinflammatory agent. Reduced neuronal degeneration after traumatic brain injury (TBI). Shown to increase histone H3 acetylation. HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration. - Chemical. CAS: 827036-76-0. Formula: C16H25N3O2S. MW: 323.5. Cell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =114nM). Displays selectivity over other HDACs (IC50=1-8microM). Neuroprotective and anti-neuroinflammatory agent. Reduced neuronal degeneration after traumatic brain injury (TBI). Shown to increase histone H3 acetylation. HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration.
- SMILESCN(C)C1=CC=C(C=C1)C(=O)NCCCCCNC(=O)CS
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Chemistry and biology of mercaptoacetamides as novel histone deacetylase inhibitors: B. Chen, et al.; Bioorg. Med. Chem. Lett. 15, 1389 (2005)
- Functional differences in epigenetic modulators - Superiority of mercaptoacetamide-based histone deacetylase inhibitors relative to hydroxamates in cortical neuron neuroprotection studies: A. Kozikowski, et al.; J. Med. Chem. 50, 3054 (2007)
- In vitro plasma stability, permeability and solubility of mercaptoacetamide histone deacetylase inhibitors: R. Konsoula & M. Jung; Int. J. Pharm. 361, 19 (2008)
- HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats: B. Zhang, et al.; Brain Res. 1226, 181 (2008)
- Novel inhibitors of human histone deacetylase (HDAC) identified by QSAR modeling of known inhibitors, virtual screening, and experimental validation: H. Tang, et al.; J. Chem. Inf. Model 49, 461 (2009)
