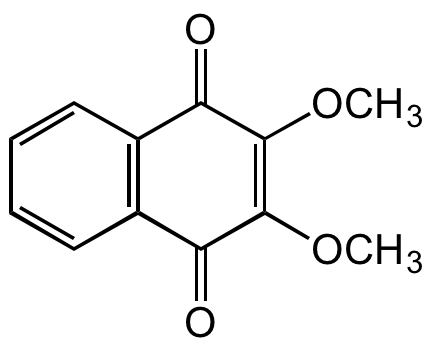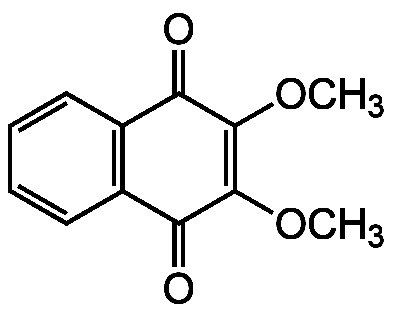
Chemical Structure
DMNQ
AG-CR1-3598
Overview
- SupplierAdipoGen Life Sciences
- Product NameDMNQ
- Delivery Days Customer10
- CAS Number6956-96-3
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationWarning
- Molecular FormulaC12H10O4
- Molecular Weight218.2
- Scientific DescriptionCell permeable, non-alkylating, non-thiol, adduct-forming, redox cycling quinone. Intracellular superoxide anion formation/ROS generation inducer. Anticancer agent. Shown to induce cell proliferation, apoptosis, necrosis and necroptosis in vitro, dependent on concentration, time, temperature and cell type. Valuable tool for the generation of reactive oxygen species (ROS) in order to study the role of ROS in cell toxicity, apoptosis and necrosis. Useful as reference compound in characterizing the effects of oxidative stress. Can be used to eliminate any mechanistic ambiguity involving redox cycling quinoids as the source of reactive oxidant species/oxidative stress in biological studies. - Chemical. CAS: 6956-96-3. Formula: C12H10O4. MW: 218.2. Cell permeable, non-alkylating, non-thiol, adduct-forming, redox cycling quinone. Intracellular superoxide anion formation/ROS generation inducer. Anticancer agent. Shown to induce cell proliferation, apoptosis, necrosis and necroptosis in vitro, dependent on concentration, time, temperature and cell type. Valuable tool for the generation of reactive oxygen species (ROS) in order to study the role of ROS in cell toxicity, apoptosis and necrosis. Useful as reference compound in characterizing the effects of oxidative stress. Can be used to eliminate any mechanistic ambiguity involving redox cycling quinoids as the source of reactive oxidant species/oxidative stress in biological studies.
- SMILESCOC1=C(OC)C(=O)C2=C(C=CC=C2)C1=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![DMNQ [6956-96-3]](https://www.targetmol.com/group3/M00/36/D8/CgoaEWayRKGEf41YAAAAAI9manM107.png)