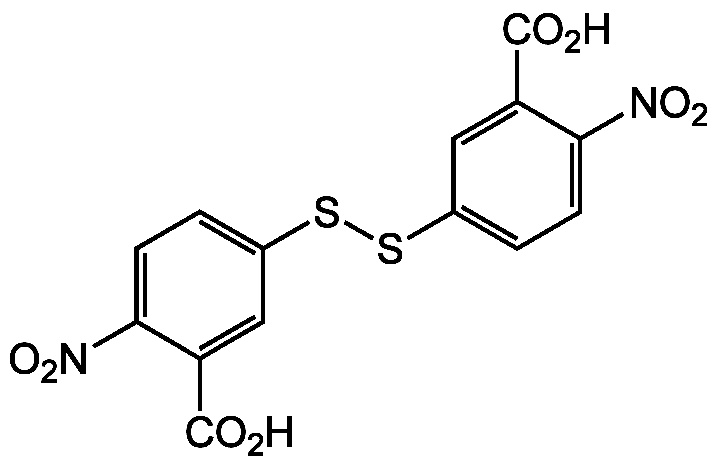
Chemical Structure
DTNB [69-78-3] [69-78-3]
CDX-D0156
CAS Number69-78-3
Product group Chemicals
Estimated Purity>98%
Molecular Weight396.35
Overview
- SupplierChemodex
- Product NameDTNB [69-78-3] [69-78-3]
- Delivery Days Customer2
- CAS Number69-78-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC14H8N2O8S2
- Molecular Weight396.35
- Scientific DescriptionA sensitive reagent for measuring the free sulfhydryl content in proteins, peptides, and tissues. Used to characterize reactive thiol groups and photometric determination of thiols and for measuring low-molecular mass thiols such as glutathione in both pure solutions and biological samples, such as blood. It can also measure the number of thiol groups on proteins. Through reaction with aliphatic thiol groups a mixed disulfide of protein thiol and one mole of 2-nitro-5-thiobenzoate per mole of protein sulfhydryl group is being formed. DTNB has little absorbance. Reaction with -SH groups on proteins (from any solvent accessible Cys) under mild alkaline conditions (pH 7-8) produces the 2-nitro-5-thiobenzoate anion, which gives an intense yellow color with an absorption maximum at 409.5nm (Extinction coefficient: 14150 M-1*cm-1). Sensitive to various buffer ions, therefore, the extinction coefficient used to calculate the number of sulfhydryl groups must be matched to the reaction conditions. In case the thiol groups are in disulfide bonds, they must be reduced under anaerobic conditions prior to reaction with DTNB. - Chemical. CAS: 69-78-3. Formula: C14H8N2O8S2. MW: 396.35. Synthetic A sensitive reagent for measuring the free sulfhydryl content in proteins, peptides, and tissues. Used to characterize reactive thiol groups and photometric determination of thiols and for measuring low-molecular mass thiols such as glutathione in both pure solutions and biological samples, such as blood. It can also measure the number of thiol groups on proteins. Through reaction with aliphatic thiol groups a mixed disulfide of protein thiol and one mole of 2-nitro-5-thiobenzoate per mole of protein sulfhydryl group is being formed. DTNB has little absorbance. Reaction with -SH groups on proteins (from any solvent accessible Cys) under mild alkaline conditions (pH 7-8) produces the 2-nitro-5-thiobenzoate anion, which gives an intense yellow color with an absorption maximum at 409.5nm (Extinction coefficient: 14150 M-1*cm-1). Sensitive to various buffer ions, therefore, the extinction coefficient used to calculate the number of sulfhydryl groups must be matched to the reaction conditions. In case the thiol groups are in disulfide bonds, they must be reduced under anaerobic conditions prior to reaction with DTNB.
- SMILESOC(=O)C1=CC(SSC2=CC=C(C(=C2)C(O)=O)[N+]([O-])=O)=CC=C1[N+]([O-])=O
- Storage InstructionRT
- UNSPSC12352200


![DTNB [69-78-3] [69-78-3]](https://www.targetmol.com/group3/M00/03/8A/CgoaEWY7TzmEQWkSAAAAACsTguw064.png)