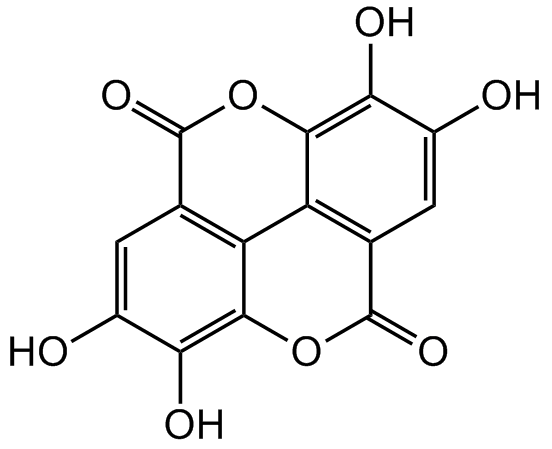
Chemical Structure
Ellagic acid [476-66-4] [476-66-4]
CDX-E0232
CAS Number476-66-4
Product group Chemicals
Estimated Purity>95%
Molecular Weight302.19
Overview
- SupplierChemodex
- Product NameEllagic acid [476-66-4] [476-66-4]
- Delivery Days Customer2
- CAS Number476-66-4
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC14H6O8
- Molecular Weight302.19
- Scientific DescriptionChemical. CAS: 476-66-4. Formula: C14H6O8. MW: 302.19. Ellagic acid is a polyphenolic antioxidant that is abundant in many fruits, vegetables, plant bark and peels. It has antioxidant, anticancer, neuroprotective, antimutagenic, anti-inflammatory and organ-preserving properties. It alters cytochrome P450 activity and improvea metabolism and clearance of xenobiotics, as well as altera immune function. Selective, ATP-competitive inhibitor of casein kinase 2 (CK2). Ellagic acid also blocks methylation of arginine 17 of histone 3 (H3R17) by coactivator-associated arginine methyltransferase 1 (CARM1) without significantly altering histone acetylase or DNA methyltransferase activity. It acts as a scavenger of oxygen species produced by hydrogen peroxide treatment and as a protector of the DNA double helix from alkylating agent injury. Also reported to inhibit topoisomerases I and II and gyrase and glutathione S-transferase. - Ellagic acid is a polyphenolic antioxidant that is abundant in many fruits, vegetables, plant bark and peels. It has antioxidant, anticancer, neuroprotective, antimutagenic, anti-inflammatory and organ-preserving properties. It alters cytochrome P450 activity and improvea metabolism and clearance of xenobiotics, as well as altera immune function. Selective, ATP-competitive inhibitor of casein kinase 2 (CK2). Ellagic acid also blocks methylation of arginine 17 of histone 3 (H3R17) by coactivator-associated arginine methyltransferase 1 (CARM1) without significantly altering histone acetylase or DNA methyltransferase activity. It acts as a scavenger of oxygen species produced by hydrogen peroxide treatment and as a protector of the DNA double helix from alkylating agent injury. Also reported to inhibit topoisomerases I and II and gyrase and glutathione S-transferase.
- SMILESOC1=CC2=C3C(OC(C4=C3C(OC2=O)=C(O)C(O)=C4)=O)=C1O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Ellagic acid [476-66-4]](https://bpsbioscience.com/media/catalog/product/2/7/27219_structure.png)


![Ellagic acid [476-66-4]](https://www.targetmol.com/group3/M00/03/2A/CgoaEGY7SLmEPq3wAAAAAOI16vo995.png)