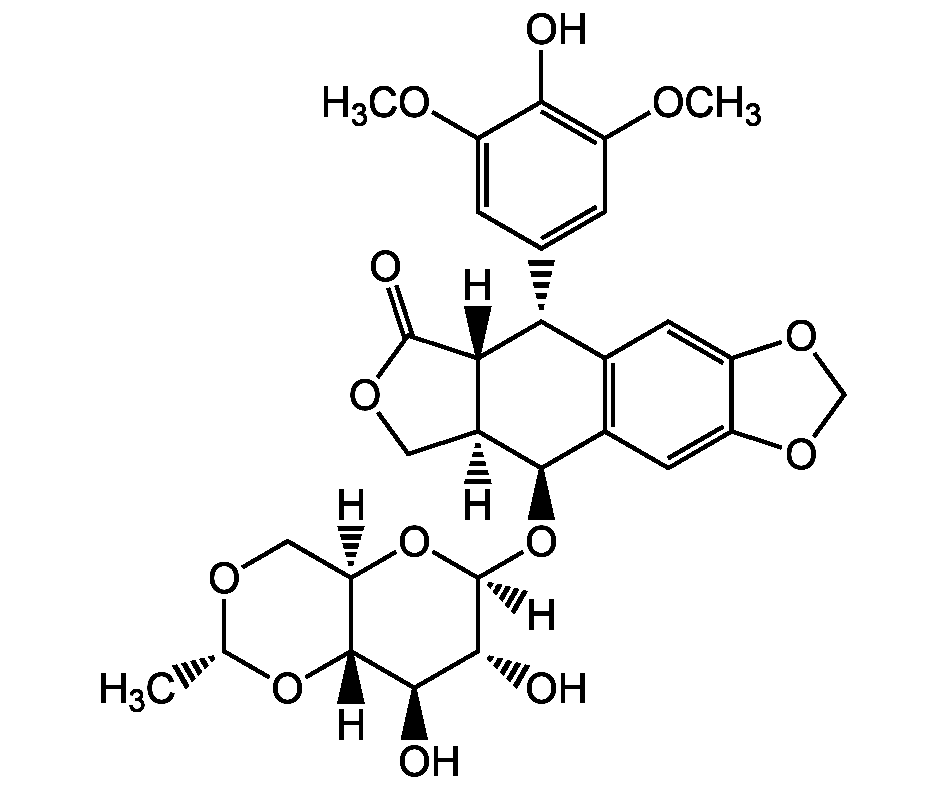
Chemical Structure
Etoposide [33419-42-0]
AG-CR1-3572
CAS Number33419-42-0
Product group Chemicals
Estimated Purity>98%
Molecular Weight588.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameEtoposide [33419-42-0]
- Delivery Days Customer10
- CAS Number33419-42-0
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger
- Molecular FormulaC29H32O13
- Molecular Weight588.6
- Scientific DescriptionChemical. CAS: 33419-42-0. Formula: C29H32O13. MW: 588.6. Semisynthetic derivative of podophyllotoxin. Potent anti-cancer compound. Induces apoptosis in normal and tumor cell lines. DNA Topoisomerase II activity inhibitor. Increases Topo II-mediated DNA breakage primarily by inhibiting the ability of the enzyme to religate cleaved nucleic acid molecules. Does not lead to immediate block of DNS synthesis, induces a progressive inhibition of DNA replication. p53 activator. Blocks the cell cycle between the end of the S phase and the early G2 phase. Oncoprotein Mdm2 synthesis inhibitor. Apoptosis inducer through the cytochrome c/Apaf-1/caspase-9 pathway and the Fas-mediated death signaling pathway. Cell cycle checkpoint activator. Affects gene expression at different levels (chromatin remodeling, transcription and alternative splicing). Chemotherapeutic compound used in cancers. Used in conditioning regimen prior to a bone marrow or blood stem cell transplantation. Highly effective in mobilizing stem cells - Potent anti-cancer compound. Induces apoptosis in normal and tumor cell lines [1, 5]. DNA Topoisomerase II activity inhibitor. Increases Topo II-mediated DNA breakage primarily by inhibiting the ability of the enzyme to religate cleaved nucleic acid molecules. Does not lead to immediate block of DNS synthesis, induces a progressive inhibition of DNA replication [2, 4, 6]. p53 activator [3]. Blocks the cell cycle between the end of the S phase and the early G2 phase [2, 8]. Oncoprotein Mdm2 synthesis inhibitor [7]. Apoptosis inducer through the cytochrome c/Apaf-1/caspase-9 pathway and the Fas-mediated death signaling pathway [9, 10]. Cell cycle checkpoint activator. Affects gene expression at different levels (chromatin remodeling, transcription and alternative splicing) [11, 14, 15]. Chemotherapeutic compound used in cancers [12, 13]. Used in conditioning regimen prior to a bone marrow or blood stem cell transplantation [16]. Highly effective in mobilizing stem cells [17]
- SMILES[H][C@]12COC(=O)[C@]1([H])[C@H](C1=CC(OC)=C(O)C(OC)=C1)C1=C(C=C3OCOC3=C1)[C@H]2O[C@]1([H])O[C@]2([H])CO[C@@H](C)O[C@@]2([H])[C@H](O)[C@H]1O
- Storage InstructionRT
- UNSPSC12352200


![Etoposide [33419-42-0]](https://www.targetmol.com/group3/M00/02/30/CgoaEGY7K5OEeKYMAAAAANr0wo0512.png)