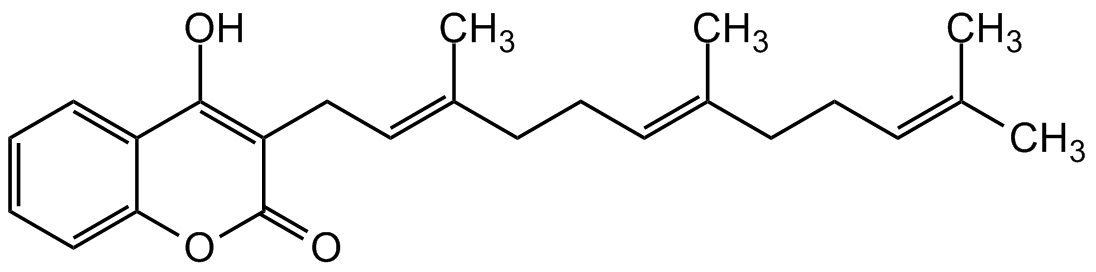
Chemical Structure
Ferulenol [6805-34-1]

AG-CN2-0011
Overview
- SupplierAdipoGen Life Sciences
- Product NameFerulenol [6805-34-1]
- Delivery Days Customer10
- CAS Number6805-34-1
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC24H30O3
- Molecular Weight366.5
- Scientific DescriptionChemical. CAS: 6805-34-1. Formula: C24H30O3. MW: 366.5. Isolated from Ferula communis. Prenylated 4-hydroxycoumarin. Anti-tumor compound. Cytotoxic. Stimulator of tubulin polymerization in vitro. Inhibitor of colchicine binding to tubulin. Antitubercular antibiotic with potent antibacterial activity. Anti-coagulant, pro-haemorrhagic compound with higher activity than warfarin. Shows hepatocyte toxicity. Disrupts mitochondrial membrane potential. - Prenylated 4-hydroxycoumarin. Anti-tumor compound [2]. Cytotoxic [2]. Stimulator of tubulin polymerization in vitro [2]. Inhibitor of colchicine binding to tubulin [2]. Antitubercular antibiotic with potent antibacterial activity [3]. Anti-coagulant, pro-haemorrhagic compound with higher activity than warfarin [4]. Shows hepatocyte toxicity [1, 4]. Disrupts mitochondrial membrane potential [5, 6]. Potent L-malate:quinone oxidoreductase (PfMQO) inhibitor in Plasmodium falciparum. Antimalarial.
- SMILESOC(C1=CC=CC=C1O2)=C(C/C=C(C)/CC/C=C(C)/CC/C=C(C)/C)C2=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Acute toxicity of ferulenol, a 4-hydroxycoumarin isolated from Ferula communis L: O. Fraigui, et al.; Vet. Hum. Toxicol. 44, 5 (2002)
- Microtubule-interacting activity and cytotoxicity of the prenylated coumarin ferulenol: C. Bocca, et al.; Planta Med. 68, 1135 (2002)
- Antimycobacterial coumarins from the sardinian giant fennel (Ferula communis): G. Appendino, et al.; J. Nat. Prod. 67, 210 (2004)
- Characterization of anti-coagulant properties of prenylated coumarin ferulenol: M. Monti, et al.; Biochim. Biophys. Acta 1770, 1437 (2007)
- Ferulenol specifically inhibits succinate ubiquinone reductase at the level of the ubiquinone cycle: M. Lahouel, et al.; BBRC 355, 252 (2007)
- Disruption of mitochondrial membrane potential by ferulenol and restoration by propolis extract: antiapoptotic role of propolis: B.H. Nadia, et al.; Acta Biol. Hung. 60, 385 (2009)
