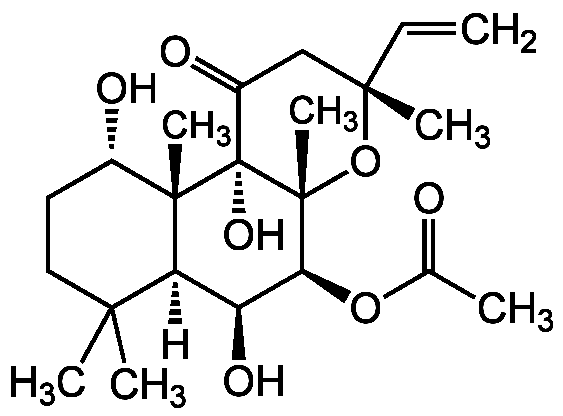
Chemical Structure
Forskolin [66428-89-5]

AG-CN2-0089
Overview
- SupplierAdipoGen Life Sciences
- Product NameForskolin [66428-89-5, 66575-29-9]
- Delivery Days Customer10
- CAS Number66428-89-5
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationWarning
- Molecular FormulaC22H34O7
- Molecular Weight410.5
- Scientific DescriptionChemical. CAS: 66428-89-5 and 66575-29-9. Formula: C22H34O7. MW: 410.5. Isolated from Coleus forskohlii. Potent, cell permeable adenylyl cyclase activator. Increases intracellular cAMP levels. Widely used tool to investigate cAMP as a second messenger. Inotropic and antihypertensive. Smooth muscle relaxant/vasodilator. Glucose transporter inhibitor. Platelet aggregation inhibitor. Stimulates lipolysis in fat cells. Non-competitive nicotinic acetylcholine receptors inhibitor. MAP kinase inhibitor. Upregulates mitochondrial uncoupling protein (UCP) mRNA levels in brown adipose tissue. Autophagy inhibitor. Hedgehog signaling inhibitor. Has antiglaucoma potential. Promotes neuronal differentiation of NSCs. - Potent, cell permeable adenylyl cyclase activator. Increases intracellular cAMP levels [1, 2, 6]. Widely used tool to investigate cAMP as a second messenger [15]. Inotropic and antihypertensive. Smooth muscle relaxant/vasodilator [3, 4, 6]. Glucose transporter inhibitor [4]. Platelet aggregation inhibitor [3, 10]. Stimulates lipolysis in fat cells [8]. Non-competitive nicotinic acetylcholine receptors inhibitor [9]. MAP kinase inhibitor [11]. Upregulates mitochondrial uncoupling protein (UCP) mRNA levels in brown adipose tissue [12]. Autophagy inhibitor [13]. Hedgehog signaling inhibitor [14]. Has antiglaucoma potential [16]. Promotes neuronal differentiation of NSCs [17]. Anti-neuroinflammatory and neuroprotective agent. Ameliorated Alzheimers disease symptoms, restoring impairment in nesting ability and sociability, and reducing neuroinflammation and amyloid beta deposition [18].
- SMILES[H][C@@]12[C@H](O)[C@H](OC(C)=O)[C@@]3(C)O[C@](C)(CC(=O)[C@]3(O)[C@@]1(C)[C@@H](O)CCC2(C)C)C=C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- The positive inotropic-acting forskolin, a potent adenylate cyclase activator: H. Metzger & E. Lindner; Arzneimittelforschung 31, 1248 (1981)
- Forskolin: a unique diterpene activator of cyclic AMP-generating systems: K.B. Seamon & J.W. Daly; J. Cyclic Nucleotide Res. 7, 201 (1981)
- Forskolin: a labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties: N.J. de Souza, et al.; Med. Res. Rev. 3, 201 (1983)
- Effects of forskolin on cerebral blood flow: Implications for a role of adenylate cyclase: D.G. Wysham, et al.; Stroke 17, 1299 (1986)
- Forskolin inhibits insulin-stimulated glucose transport in rat adipose cells by a direct interaction with the glucose transporter: H.G. Joost & H.J. Steinfelder; Mol. Pharmacol. 31, 279 (1987)
- Effect of forskolin on cytosolic Ca++ level and contraction in vascular smooth muscle: A. Abe & H. Karaki; J. Pharmacol. Exp. Ther. 249, 895 (1989)
- Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action?: A. Laurenza, et al.; TIPS 10, 442 (1989) (Review)
- Relationship between cyclic AMP production and lipolysis induced by forskolin in rat fat cells: H. Okuda, et al.; J. Lipid Res. 33, 225 (1992)
- Forskolin acts as a noncompetitive inhibitor of nicotinic acetylcholine receptors: M.L. Aylwin & M.M. White; Mol. Pharmacol. 41, 908 (1992)
- Forskolin inhibits platelet-activating factor binding to platelet receptors independently of adenylyl cyclase activation: S. Wong, et al.; Eur. J. Pharmacol. 245, 55 (1993)
