Chemical Structure
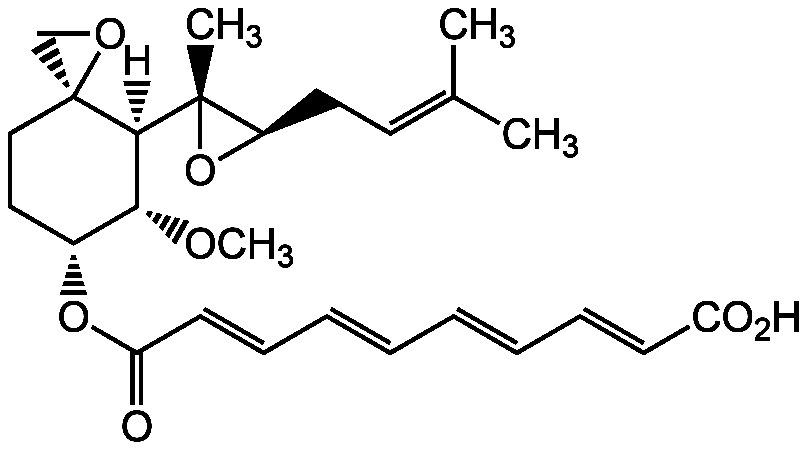
Fumagillin [23110-15-8]

AG-CN2-0529
Overview
- SupplierAdipoGen Life Sciences
- Product NameFumagillin [23110-15-8]
- Delivery Days Customer10
- CAS Number23110-15-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC26H34O7
- Molecular Weight458.6
- Scientific DescriptionChemical. CAS: 23110-15-8. Formula: C26H34O7. MW: 458.6. Isolated from Aspergillus fumigatus. Meroterpenoid antibiotic. Anticancer, antimicrobial, antimalarial and amoebicidal compound. Potent, selective and covalent inhibitor of methionine aminopeptidase-2 (MetAP2). Anti-angiogenic by impairing the growth of endothelial cells and altering gene expression. Inhibits endothelial cell proliferation in vitro and tumor-induced angiogenesis in vivo. Suppresses the HIV-1 infection of human macrophages through the inhibition of HIV-1 viral protein R (Vpr) activity. Inhibits neovascularization and might be useful in non-tumor diseases such as diabetic retinopathy, arthritis and psoriasis, which involve neovascularisation processes. Reduces diet-induced adipose tissue formation in mice, independent of its effects on angiogenesis. - Meroterpenoid antibiotic. Anticancer, antimicrobial, antimalarial and amoebicidal compound. Potent, selective and covalent inhibitor of methionine aminopeptidase-2 (MetAP2). Anti-angiogenic by impairing the growth of endothelial cells and altering gene expression. Inhibits endothelial cell proliferation in vitro and tumor-induced angiogenesis in vivo. Suppresses the HIV-1 infection of human macrophages through the inhibition of HIV-1 viral protein R (Vpr) activity. Inhibits neovascularization and might be useful in non-tumor diseases such as diabetic retinopathy, arthritis and psoriasis, which involve neovascularisation processes. Reduces diet-induced adipose tissue formation in mice, independent of its effects on angiogenesis.
- SMILES[H][C@@]1([C@H](OC)[C@@H](CC[C@]11CO1)OC(=O)\C=C\C=C\C=C\C=C\C(O)=O)[C@@]1(C)O[C@@H]1CC=C(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
