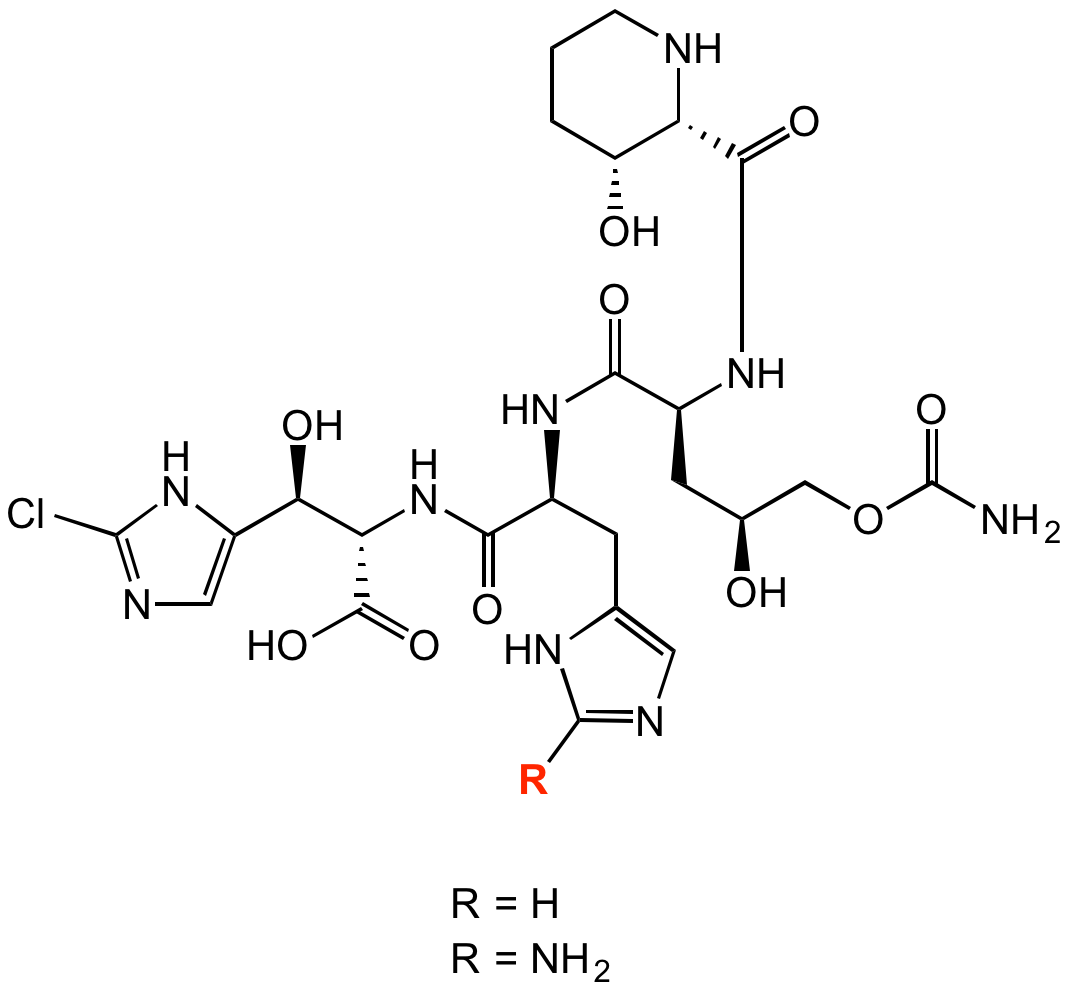
Chemical Structure
GE81112 A/B [883726-13-4] [883726-13-4]
AG-CN2-0306
CAS Number883726-13-4
Product group Chemicals
Estimated Purity>90%
Molecular Weight644.0 [A]659.1 [B]
Overview
- SupplierAdipoGen Life Sciences
- Product NameGE81112 A/B [883726-13-4] [883726-13-4]
- Delivery Days Customer10
- CAS Number883726-13-4
- CertificationResearch Use Only
- Estimated Purity>90%
- Molecular FormulaC24H34ClN9O10 [A] C24H35ClN10O10 [B]
- Molecular Weight644.0 [A]659.1 [B]
- Scientific DescriptionChemical. CAS: 883726-13-4 [GE81112A], 883726-14-5 [GE81112B]. Formula: C24H34ClN9O10 [A], C24H35ClN10O10 [B]. MW: 644.0 [A]659.1 [B]. Isolated from Strepomyces sp. Tetrapeptide antibiotic. Potent and selective inhibitor of bacterial protein synthesis. Translational inhibitor specific for the initiation phase. Binds to the 30S ribosomal subunit and specifically inhibits P-site decoding of the mRNA initiation codon by the fMet-tRNA anticodon. Inhibits in vivo protein synthesis but not other cell functions such as DNA duplication, transcription and cell wall synthesis. Antibacterial activity against some Gram-positive and Gram-negative bacteria. Unique scaffold for designing new drugs. - Tetrapeptide antibiotic. Potent and selective inhibitor of bacterial protein synthesis. Translational inhibitor specific for the initiation phase. Binds to the 30S ribosomal subunit and specifically inhibits P-site decoding of the mRNA initiation codon by the fMet-tRNA anticodon. Inhibits in vivo protein synthesis but not other cell functions such as DNA duplication, transcription and cell wall synthesis. Antibacterial activity against some Gram-positive and Gram-negative bacteria. Unique scaffold for designing new drugs.
- SMILESR=H: O[C@H](C1=CN=C(Cl)N1)[C@@H](C(O)=O)NC([C@@H](NC([C@H](C[C@H](O)COC(N)=O)NC([C@@H]2[C@@H](CCCN2)O)=O)=O)CC3=CN=C([H])N3)=O R=NH2: O[C@H](C1=CN=C(Cl)N1)[C@@H](C(O)=O)NC([C@@H](NC([C@H](C[C@H](O)COC(N)=O)NC([C@@H]2[C@@H](CCCN2)O)=O)=O)CC3=CN=C(N)N3)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
