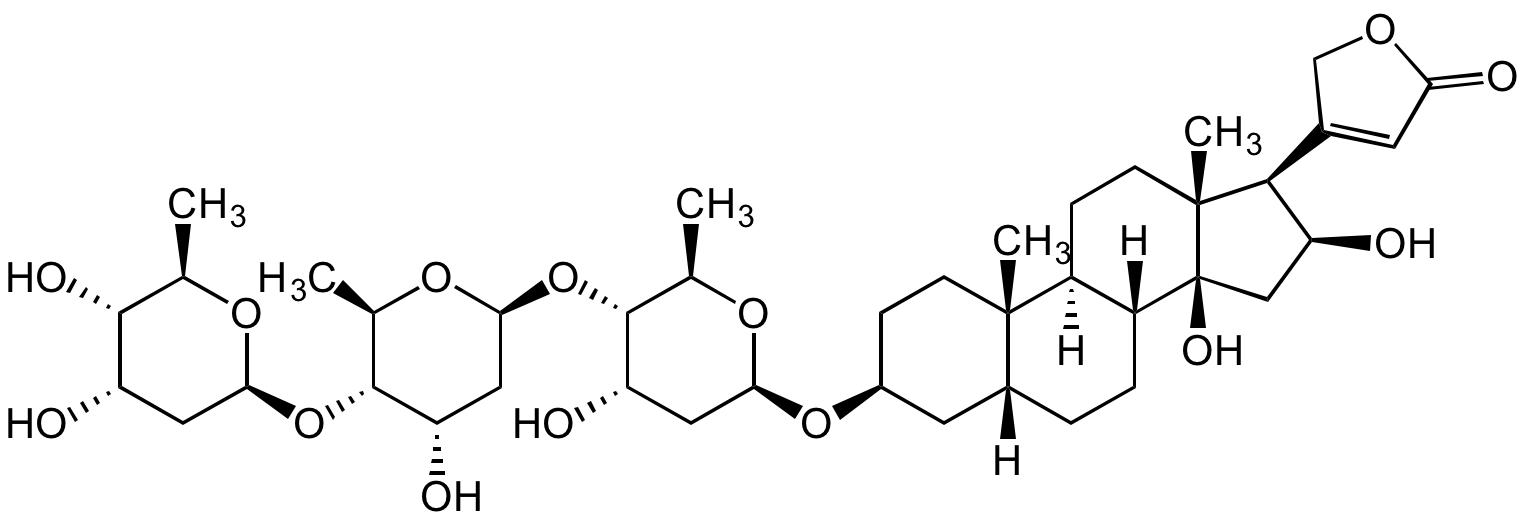
Chemical Structure
Gitoxin [4562-36-1]

AG-CN2-0485
Overview
- SupplierAdipoGen Life Sciences
- Product NameGitoxin [4562-36-1]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number4562-36-1
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC41H64O14
- Molecular Weight780.9
- Scientific DescriptionCardiac glycoside. Natural metabolite of digitoxin. Starting material for the synthesis of gitoxin derivatives with activity as cardiac glycosides. Uncompetitive inhibitor of the Na+/K+ ATPase activity. Inhibits growth of selected human cancer cells. - Chemical. CAS: 4562-36-1. Formula: C41H64O14. MW: 780.9. Isolated from Digitalis purpurea L. Cardiac glycoside. Natural metabolite of digitoxin. Starting material for the synthesis of gitoxin derivatives with activity as cardiac glycosides. Uncompetitive inhibitor of the Na+/K+ ATPase activity. Inhibits growth of selected human cancer cells.
- SMILESO[C@@H]1[C@H](O)[C@@H](C)O[C@@H](O[C@H]([C@@H](C)O[C@@H](O[C@H]([C@@H](C)O[C@@H](O[C@H](C[C@@]2([H])CC[C@]3([H])[C@]4([H])CC[C@@]5([C@H]([C@@H](O)C[C@]35O)C(CO6)=CC6=O)C)CC[C@@]24C)C7)[C@H]7O)C8)[C@H]8O)C1
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200
References
- Studies on digitalis glycosides. VII. Gitoroside, digitalonin, and gitoxin pentaacetate: D. Satoh, et al.; Pharm. Bull. 5, 253 (1957)
- Effects of digoxin and gitoxin on the enzymatic activity and kinetic parameters of Na+/K+-ATPase: D. Krstic, et al.; J. Enzyme Inhib. Med. Chem. 19, 409 (2004)
- Na+/K+-ATPase: Activity and inhibition: M. Colovic, et al.; Russ. J. Phys. Chem. A. 83, 1602 (2009)
- A structural view on the functional importance of the sugar moiety and steroid hydroxyls of cardiotonic steroids in binding to Na,K-ATPase: F. Cornelius, et al.; J. Biol. Chem. 288, 6602 (2013)
- Hellebrin and its aglycone form hellebrigenin display similar in vitro growth inhibitory effects in cancer cells and binding profiles to the alpha subunits of the Na+/K+-ATPase: L. Moreno Y. Banuls, et al.; Mol. Cancer 12, 33 (2013)
