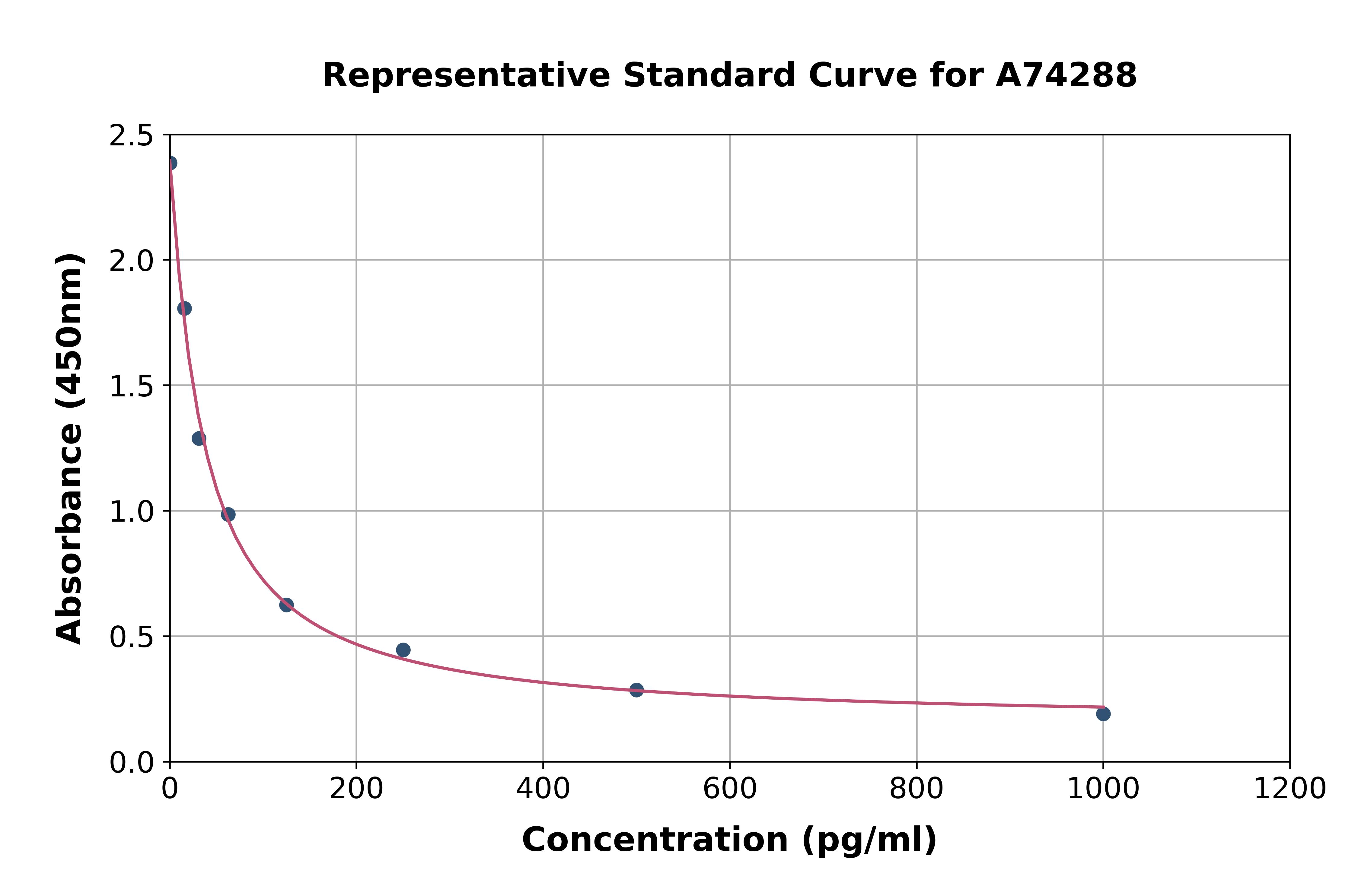
Glicentin ELISA Human
10-1273-01
Assay Sample TypeSerum or EDTA plasma
Product group Assays
Overview
- SupplierMercodia
- Product NameGlicentin ELISA Human
- Delivery Days Customer5
- ApplicationsELISA
- Assay Detection Range3 - 300 pmol/L (24-2400 pg/mL)
- Assay Sample TypeSerum or EDTA plasma
- Assay Sensitivity3 pmol/L
- Assay TimeIncubation (min) : 120 + 15
- CertificationResearch Use Only
- Scientific DescriptionMercodia Glicentin ELISA is a high-quality immunoassay for the quantification of human glicentin in serum, plasma and cell culture media. Glicentin is a proglucagon derived gut peptide that is released in the gut in response to feed intake. It is present in high levels in the circulation after stimulation and is often a problem when measuring glucagon and other proglucagon derivates because of it’s shared sequence. The Mercodia Glicentin ELISA however, has no cross-reactivity to such circulating proglucagon derived gut peptides. • Highly specific, sensitive and rapid method for determination of glicentin • No cross-reactivity to circulating proglucagon derived gut peptides • Assay buffers and calibrators have been optimized to prevent degradation and aggregation of glicentin
- UNSPSC41116133
References
- Raffort J, Lareyre F, Massalou D, et al. Insights on glicentin, a promising peptide of the proglucagon family. Biochem Med (Zagreb). 2017,27(2):308-324. doi: 10.11613/BM.2017.034Read this paper
- Raffort J, Panaïa-Ferrari P, Lareyre F, et al. Fasting Circulating Glicentin Increases After Bariatric Surgery. Obes Surg. 2017,27(6):1581-1588. doi: 10.1007/s11695-016-2493-5Read this paper
- Manell H, Staaf J, Manukyan L, et al. Altered Plasma Levels of Glucagon, GLP-1 and Glicentin During OGTT in Adolescents With Obesity and Type 2 Diabetes. J Clin Endocrinol Metab. 2016,101(3):1181-9. doi: 10.1210/jc.2015-3885Read this paper