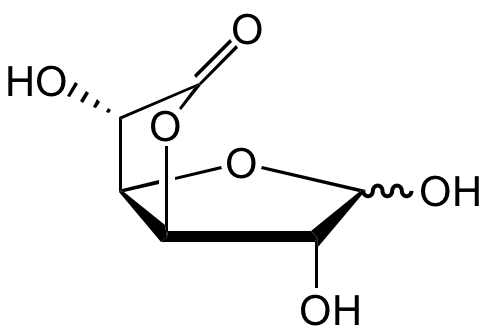
Chemical Structure
Glucuronolactone [32449-92-6]
CDX-G0044
CAS Number32449-92-6
Product group Chemicals
Estimated Purity>98%
Molecular Weight176.12
Overview
- SupplierChemodex
- Product NameGlucuronolactone [32449-92-6]
- Delivery Days Customer2
- CAS Number32449-92-6
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC6H8O6
- Molecular Weight176.12
- Scientific DescriptionChemical. CAS: 32449-92-6. Formula: C6H8O6. MW: 176.12. Synthetic. Naturally occurring carbohydrate derivative that is an important structural component of nearly all connective tissues and is also found in many plant gums. Metabolized to glucaric acid, xylitol, and L-xylulose, and humans may also be able to use glucuronolactone as a precursor for ascorbic acid synthesis. Used as a detoxicant. The liver uses glucose to create glucuronolactone, which inhibits the enzyme B-glucuronidase (metabolizes glucuronides), which should cause blood-glucuronide levels to rise. Glucuronides combine with toxic substances, such as morphine and depot medroxyprogesterone acetate, by converting them to water-soluble glucuronide-conjugates which are excreted in the urine. Used as building block and starting reagent for synthesis of drugs, optically active glucopyranoses and long-chain alkyl glucofuranosides. - Naturally occurring carbohydrate derivative that is an important structural component of nearly all connective tissues and is also found in many plant gums. Metabolized to glucaric acid, xylitol, and L-xylulose, and humans may also be able to use glucuronolactone as a precursor for ascorbic acid synthesis. Used as a detoxicant. The liver uses glucose to create glucuronolactone, which inhibits the enzyme B-glucuronidase (metabolizes glucuronides), which should cause blood-glucuronide levels to rise. Glucuronides combine with toxic substances, such as morphine and depot medroxyprogesterone acetate, by converting them to water-soluble glucuronide-conjugates which are excreted in the urine. Used as building block and starting reagent for synthesis of drugs, optically active glucopyranoses and long-chain alkyl glucofuranosides.
- SMILESO[C@@H]1[C@@H](OC2=O)[C@@H]([C@@H]2O)OC1O
- Storage InstructionRT
- UNSPSC12352200

![D-Glucuronic acid lactone [32449-92-6]](https://www.targetmol.com/group3/M00/03/5A/CgoaEGY7TxSEPo2nAAAAAKX9xSU989.png)