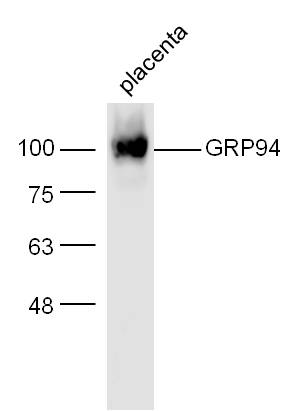
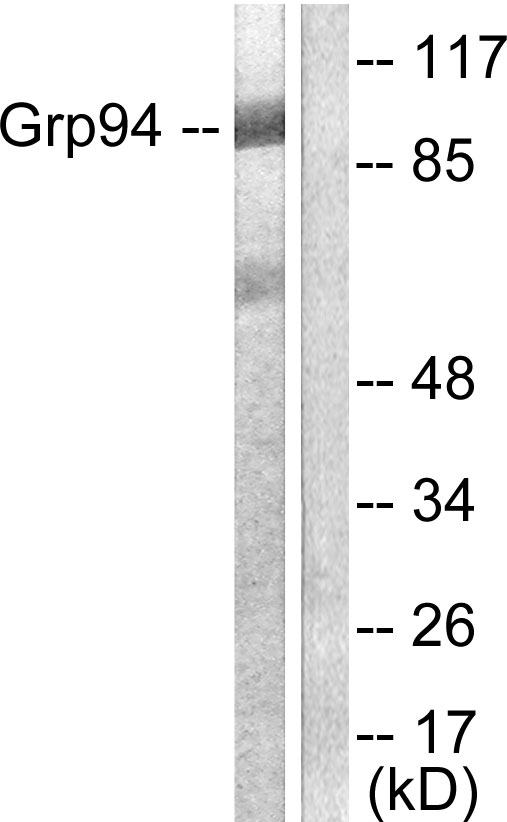
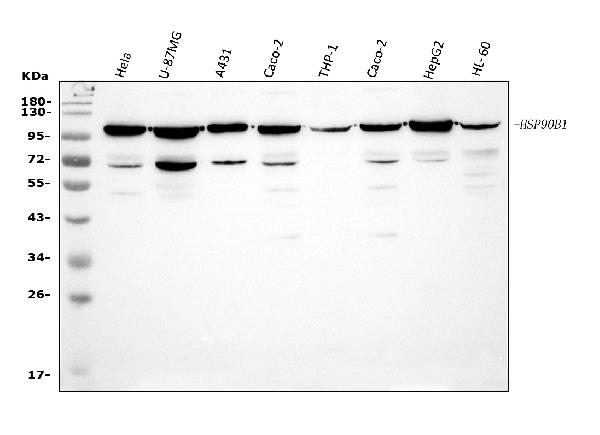
GRP94 Antibody (OASE00005)
OASE00005
ApplicationsFlow Cytometry, ImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry
Product group Antibodies
TargetHSP90B1
Overview
- SupplierAviva Systems Biology
- Product NameGRP94 Antibody (OASE00005)
- Delivery Days Customer23
- ApplicationsFlow Cytometry, ImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone ID9G10
- Concentration1 mg/ml
- ConjugateUnconjugated
- Gene ID7184
- Target nameHSP90B1
- Target descriptionheat shock protein 90 beta family member 1
- Target synonymsECGP, GP96, GRP94, HEL-S-125m, HEL35, TRA1, endoplasmin, 94 kDa glucose-regulated protein, endothelial cell (HBMEC) glycoprotein, epididymis luminal protein 35, epididymis secretory sperm binding protein Li 125m, heat shock protein 90 kDa beta member 1, heat shock protein 90kDa beta (Grp94), member 1, heat shock protein 90kDa beta family member 1, heat shock protein family C member 4, stress-inducible tumor rejection antigen gp96, tumor rejection antigen (gp96) 1, tumor rejection antigen 1
- HostRat
- IsotypeIgG2a
- Scientific DescriptionGrp94 (glucose regulated protein 94, gp96) is a constitutively expressed endoplasmic reticulum (ER) lumenal protein that is up-regulated in response to cellular stress such as heat shock, oxidative stress or glucose depletion. Grp94 is thought to play a role in protein translocation to the ER, in their subsequent folding and assembly, and in regulating protein secretion (1). Grp94 also plays a role in antigen presentation by accessing the endogenous pathway and eliciting specific CTL responses to chaperone bound peptides via MHC class I pathway (2). Grp94 is a member of the HSP90 family of stress proteins and shares sequence homology with its cytosolic equivalent, HSP90 (3). Both HSP90 and Grp94 are calcium binding proteins (4). Despite sharing 50% sequence homology over its N domains and complete conservation in its ligand binding domains with HSP90, Grp94 and HSP90 differ in their interactions with regulatory ligands as Grp94 has weak ATP binding and hydrolysis activity (5). Grp94 exists as a homodimer and the two subunits interact at two distinct intermolecular sites, C terminal dimerization domains and the N-terminal interacts with the middle domain of opposing subunits (6). Grp94 contains a carboxy terminal KDEL (Lys-Asp-Glu-Leu) sequence which is believed to aid in its retention in the ER (7).
- Storage Instruction-20°C
- UNSPSC12352203