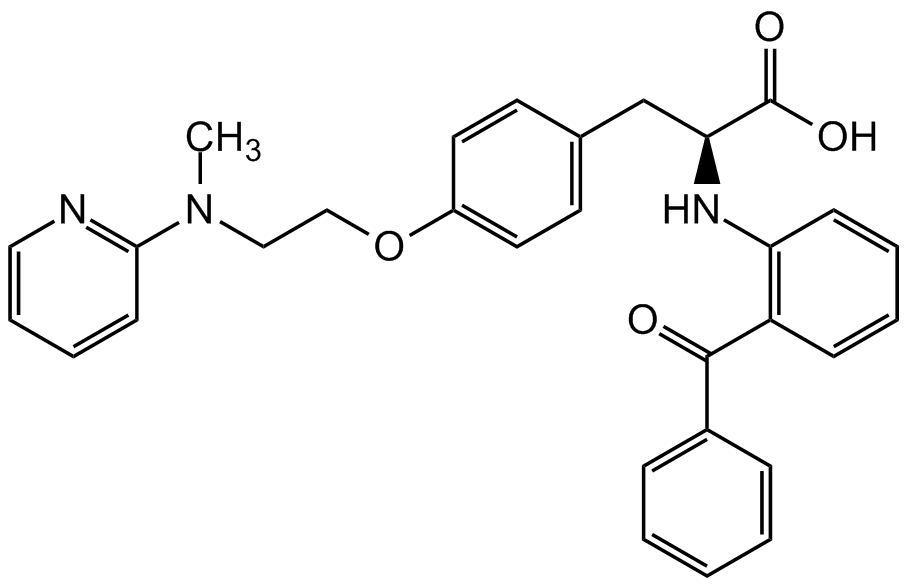
Chemical Structure
GW1929 [196808-24-9]

AG-CR1-0116
Overview
- SupplierAdipoGen Life Sciences
- Product NameGW1929 [196808-24-9]
- Delivery Days Customer10
- CAS Number196808-24-9
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC30H29N3O4
- Molecular Weight495.6
- Scientific DescriptionChemical. CAS: 196808-24-9. Formula: C30H29N3O4. MW: 495.6. Potent and subtype-selective (>1000-fold) PPARgamma agonist. Angiogenesis inhibitor. Apoptosis inhibitor. Anti-inflammatory. Anti-hyperglycemic and anti-hyperlipidemic agent. Antidiabetic. The glucose-lowering effect in rats is 100-fold more potent than that of troglitazone. - Potent and subtype-selective (>1000-fold) PPARgamma agonist [1]. Angiogenesis inhibitor [3]. Apoptosis inhibitor [4]. Anti-inflammatory [4]. Anti-hyperglycemic and anti-hyperlipidemic agent [1]. Antidiabetic. The glucose-lowering effect in rats is 100-fold more potent than that of troglitazone [2].
- SMILESCOC1=CC(=O)OC(=C1)C1C2(O)CC3OC(=O)[C@]2(O)C(C(=O)C2=CC=CC=C2)C1(O)C3O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- N-(2-Benzoylphenyl)-L-tyrosine PPARgamma agonists. 1. Discovery of a novel series of potent antihyperglycemic and antihyperlipidemic agents: B.R. Henke, et al.; J. Med. Chem. 41, 5020 (1998)
- A novel N-aryl tyrosine activator of peroxisome proliferator-activated receptor-gamma reverses the diabetic phenotype of the Zucker diabetic fatty rat: K.K. Brown, et al.; Diabetes 48, 1415 (1999)
- PPARgamma agonists inhibit angiogenesis by suppressing PKCalpha- and CREB-mediated COX-2 expression in the human endothelium: E. Scoditti, et al.; Cardiovasc. Res. 86, 302 (2010)
- Ameliorative Effects of GW1929, a Nonthiazolidinedione PPAR gamma Agonist, on Inflammation and Apoptosis in Focal Cerebral Ischemic-Reperfusion Injury: R.K. Kaundal & S.S. Sharma; Curr. Neurovasc. Res. 8, 236 (2011)
