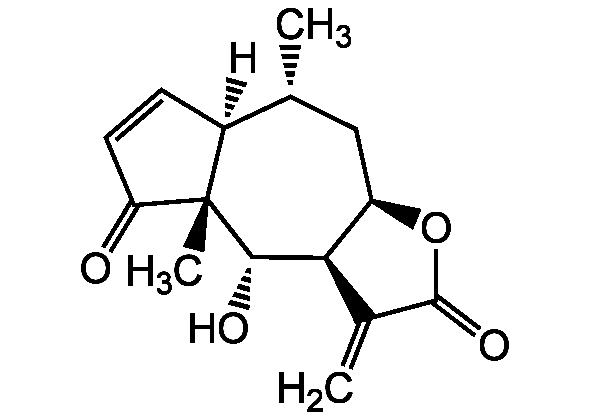
Chemical Structure
Helenalin [6754-13-8]

AG-CN2-0435
Overview
- SupplierAdipoGen Life Sciences
- Product NameHelenalin [6754-13-8]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number6754-13-8
- CertificationResearch Use Only
- Estimated Purity>96%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC15H18O4
- Molecular Weight262.3
- Scientific DescriptionAnticancer compound. NF-kappaB inhibitor. Apoptosis inducer. Potent anti-inflammatory agent. Telomerase inhibitor. Anti-trypanosomal and antiprotozoal compound Antibiotic. Shows anti-proliferative effects in 3T3-L1 preadipocytes. Autophagy inducer. - Chemical. CAS: 6754-13-8. Formula: C15H18O4. MW: 262.3. Isolated from Arnica chamissonis. Anticancer compound. NF-kappaB inhibitor. Apoptosis inducer. Potent anti-inflammatory agent. Telomerase inhibitor. Anti-trypanosomal and antiprotozoal compound Antibiotic. Shows anti-proliferative effects in 3T3-L1 preadipocytes. Autophagy inducer.
- SMILES[H][C@@]12C=CC(=O)[C@@]1(C)[C@@H](O)[C@H]1[C@@H](C[C@H]2C)OC(=O)C1=C
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 3462
- UNSPSC12352200
References
- Constituents of Helenium species.XIII. The structure of Helenalin and Mexicanin A: W. Herz, et al.; JACS 85, 19 (1963)
- Antitumor agents. 21. A proposed mechanism for inhibition of cancer growth by tenulin and helenalin and related cyclopentenones: I.H. Hall, et al.; J. Med. Chem. 20, 333 (1977)
- Inhibition of nucleic acid synthesis in P-388 lymphocytic leukemia tumor cells by helenalin and bis(helenalinyl)malonate in vivo: W.L. Williams Jr, et al.; J. Pharm. Sci. 77, 178 (1988)
- Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-kappaB: G. Lyss, et al.; J. Biol. Chem. 378, 951 (1997)
- The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65: G. Lyss, et al; J. Biol. Chem. 273, 33508 (1998)
- Helenalin triggers a CD95 death receptor-independent apoptosis that is not affected by overexpression of Bcl-x(L) or Bcl-2: V.M. Dirsch, et al.; Cancer Res. 61, 5817 (2001)
- Anti-trypanosomal activity of helenalin and some structurally related sesquiterpene lactones: T.J. Schmidt, et al.; Planta Med. 68, 750 (2002)
- Induction of human leukemia HL-60 cell differentiation via a PKC/ERK pathway by helenalin, a pseudoguainolide sesquiterpene lactone: S.H. Kim, et al.; Eur. J. Pharmacol. 511, 89 (2005)
- Potent inhibition of human telomerase by helenalin: P.R. Huang, et al.; Cancer Lett. 227, 169 (2005)
- Novel effect of helenalin on Akt signaling and Skp2 expression in 3T3-L1 preadipocytes: C.A. Auld, et al.; BBRC 346, 314 (2006)
