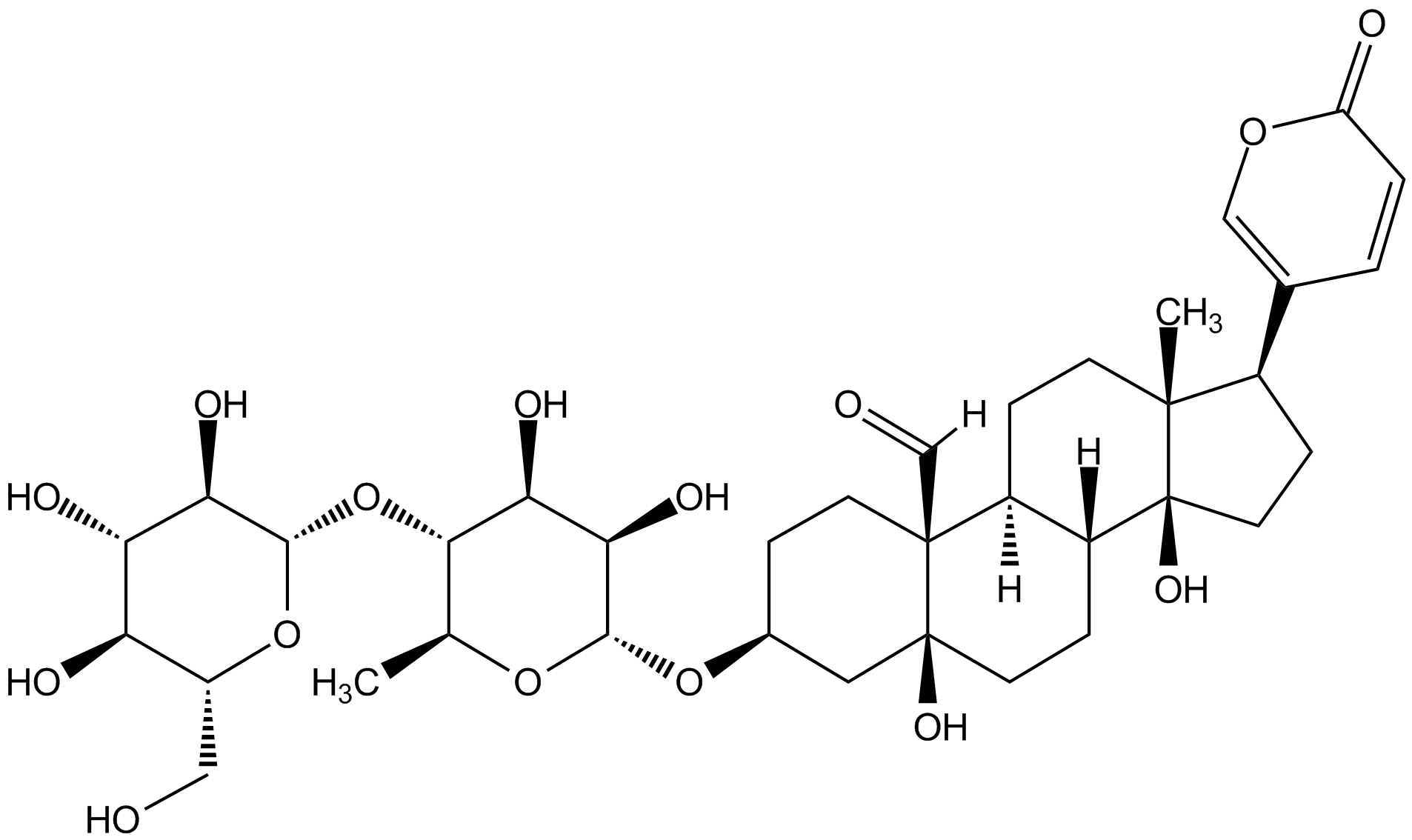
Chemical Structure
Hellebrin [13289-18-4]

AG-CN2-0475
Overview
- SupplierAdipoGen Life Sciences
- Product NameHellebrin [13289-18-4]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number13289-18-4
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC36H52O15
- Molecular Weight724.8
- Scientific DescriptionChemical. CAS: 13289-18-4. Formula: C36H52O15. MW: 724.8. Isolated from Helleborus purpurascens. Highly potent Na+/K+-ATPase inhibitor, blocking the active efflux of Na+ and reuptake of K+. Water soluble cardiotonic glycoside from the class of bufadienolides (with 2-pyrone ring). Inhibits cancer cell growth in vitro. Shown to induce caspase-dependent apoptosis, reducing MDR resistance and the rate of mitochondrial oxidative phosphorylation in cancer cells. Inotropic by increasing the intracellular Ca2+ concentration. Immunosuppressive and anti-inflammatory agent that inhibits allogeneic T cell activation with much higher potency than cortisol or cyclosporin A. - Highly potent Na+/K+-ATPase inhibitor, blocking the active efflux of Na+ and reuptake of K+. Water soluble cardiotonic glycoside from the class of bufadienolides (with 2-pyrone ring). Inhibits cancer cell growth in vitro. Shown to induce caspase-dependent apoptosis, reducing MDR resistance and the rate of mitochondrial oxidative phosphorylation in cancer cells. Inotropic by increasing the intracellular Ca2+ concentration. Immunosuppressive and anti-inflammatory agent that inhibits allogeneic T cell activation with much higher potency than cortisol or cyclosporin A. Anti-seizure activity in pentylenetetrazole (PTZ)-induced epileptical seizures.
- SMILES[H]C(=O)[C@]12CC[C@@H](C[C@@]1(O)CCC1C2CC[C@]2(C)[C@H](CC[C@]12O)C1=COC(=O)C=C1)O[C@@H]1OC(C)[C@H](O[C@@H]2OC(CO)[C@@H](O)[C@@H](O)C2O)[C@H](O)C1O
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200
References
- Ueber Hellebrin, ein krystallisiertes Glykosid aus Radix Hellebori nigri: W. Karrer; Helv. Chim. Acta 26, 1353 (1943)
- The structure of hellebrin: P. Muhr, et al.; Liebigs Ann. Org. Bioorg. Chem. 2, 443 (1995)
- In search of ideal inotropic steroids: recent progress: K.R. Repke, et al.; Prog. Drug Res. 47, 9 (1996)
- Exquisitely small amounts of nonglucocorticoid natural steroids suppress the human allogeneic T-cell response: P. Terness, et al.; Transplant Proc. 33, 547 (2001)
- The T-cell suppressive effect of bufadienolides: structural requirements for their immunoregulatory activity: P. Terness, et al.; Int. Immunopharmacol. 1, 119 (2001)
- Apoptosis-mediated selective killing of malignant cells by cardiac steroids: maintenance of cytotoxicity and loss of cardiac activity of chemically modified derivatives: D. Daniel, et al.; Int. Immunopharmacol. 3, 1791 (2003)
- Anti-cancer natural product library from traditional chinese medicine: V.B. Konkimalla & T. Efferth; Comb. Chem. High Throughput Screen. 11, 7 (2008)
- FTIR spectral signature of the effect of cardiotonic steroids with antitumoral properties on a prostate cancer cell line: R. Gasper, et al.; Biochim. Biophys. Acta 1802, 1087 (2010)
- Hellebrin and its aglycone form hellebrigenin display similar in vitro growth inhibitory effects in cancer cells and binding profiles to the alpha subunits of the Na+/K+-ATPase: L. Moreno Y. Banuls, et al.; Mol. Cancer 12, 33 (2013)
- Structure-activity relationship analysis of bufadienolide-induced in vitro growth inhibitory effects on mouse and human cancer cells: L. Moreno Y. Banuls, et al.; J. Nat. Prod. 76, 1078 (2013)
